Three-Dimensional Light Sheet Fluorescence Microscopy of Lungs To Dissect Local Host Immune-Aspergillus fumigatus Interactions
- PMID: 32019790
- PMCID: PMC7002341
- DOI: 10.1128/mBio.02752-19
Three-Dimensional Light Sheet Fluorescence Microscopy of Lungs To Dissect Local Host Immune-Aspergillus fumigatus Interactions
Abstract
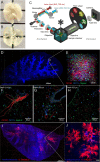
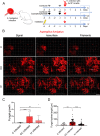
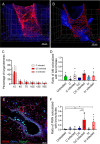
Aspergillus fumigatus is an opportunistic fungal pathogen that can cause life-threatening invasive lung infections in immunodeficient patients. The cellular and molecular processes of infection during onset, establishment, and progression of A. fumigatus infections are highly complex and depend on both fungal attributes and the immune status of the host. Therefore, preclinical animal models are of paramount importance to investigate and gain better insight into the infection process. Yet, despite their extensive use, commonly employed murine models of invasive pulmonary aspergillosis are not well understood due to analytical limitations. Here, we present quantitative light sheet fluorescence microscopy (LSFM) to describe fungal growth and the local immune response in whole lungs at cellular resolution within its anatomical context. We analyzed three very common murine models of pulmonary aspergillosis based on immunosuppression with corticosteroids, chemotherapy-induced leukopenia, or myeloablative irradiation. LSFM uncovered distinct architectures of fungal growth and degrees of tissue invasion in each model. Furthermore, LSFM revealed the spatial distribution, interaction, and activation of two key immune cell populations in antifungal defense: alveolar macrophages and polymorphonuclear neutrophils. Interestingly, the patterns of fungal growth correlated with the detected effects of the immunosuppressive regimens on the local immune cell populations. Moreover, LSFM demonstrates that the commonly used intranasal route of spore administration did not result in complete intra-alveolar deposition, as about 80% of fungal growth occurred outside the alveolar space. Hence, characterization by LSFM is more rigorous than by previously used methods employing murine models of invasive pulmonary aspergillosis and pinpoints their strengths and limitations.IMPORTANCE The use of animal models of infection is essential to advance our understanding of the complex host-pathogen interactions that take place during Aspergillus fumigatus lung infections. As in the case of humans, mice need to suffer an immune imbalance in order to become susceptible to invasive pulmonary aspergillosis (IPA), the most serious infection caused by A. fumigatus There are several immunosuppressive regimens that are routinely used to investigate fungal growth and/or immune responses in murine models of invasive pulmonary aspergillosis. However, the precise consequences of the use of each immunosuppressive model for the local immune populations and for fungal growth are not completely understood. Here, to pin down the scenarios involving commonly used IPA models, we employed light sheet fluorescence microscopy (LSFM) to analyze whole lungs at cellular resolution. Our results will be valuable to optimize and refine animal models to maximize their use in future research.
Keywords: Aspergillus fumigatus; host immune response; host-pathogen interactions; in vivo fungal growth; invasive aspergillosis; light sheet fluorescence microscopy; lung immunity; lung infection; microscopy/imaging; murine models of invasive pulmonary aspergillosis; whole-organ imaging.
Copyright © 2020 Amich et al.
Figures


References
-
- Paulussen C, Hallsworth JE, Álvarez-Pérez S, Nierman WC, Hamill PG, Blain D, Rediers H, Lievens B. 2017. Ecology of aspergillosis: insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microb Biotechnol 10:296–322. doi:10.1111/1751-7915.12367. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
