RNA-Seq Analysis Illuminates the Early Stages of Plasmodium Liver Infection
- PMID: 32019802
- PMCID: PMC7002348
- DOI: 10.1128/mBio.03234-19
RNA-Seq Analysis Illuminates the Early Stages of Plasmodium Liver Infection
Abstract
The apicomplexan parasites Plasmodium spp. are the causative agents of malaria, a disease that poses a significant global health burden. Plasmodium spp. initiate infection of the human host by transforming and replicating within hepatocytes. This liver stage (LS) is poorly understood compared to other Plasmodium life stages, which has hindered our ability to target these parasites for disease prevention. We conducted an extensive transcriptome sequencing (RNA-Seq) analysis throughout the Plasmodium berghei LS, covering as early as 2 h postinfection (hpi) and extending to 48 hpi. Our data revealed that hundreds of genes are differentially expressed at 2 hpi and that multiple genes shown to be important for later infection are upregulated as early as 12 hpi. Using hierarchical clustering along with coexpression analysis, we identified clusters functionally enriched for important liver-stage processes such as interactions with the host cell and redox homeostasis. Furthermore, some of these clusters were highly correlated to the expression of ApiAP2 transcription factors, while showing enrichment of mostly uncharacterized DNA binding motifs. This finding indicates potential LS targets for these transcription factors, while also hinting at alternative uncharacterized DNA binding motifs and transcription factors during this stage. Our work presents a window into the previously undescribed transcriptome of Plasmodium upon host hepatocyte infection to enable a comprehensive view of the parasite's LS. These findings also provide a blueprint for future studies that extend hypotheses concerning LS gene function in P. berghei to human-infective Plasmodium parasites.IMPORTANCE The LS of Plasmodium infection is an asymptomatic yet necessary stage for producing blood-infective parasites, the causative agents of malaria. Blocking the liver stage of the life cycle can prevent clinical malaria, but relatively less is known about the parasite's biology at this stage. Using the rodent model P. berghei, we investigated whole-transcriptome changes occurring as early as 2 hpi of hepatocytes. The transcriptional profiles of early time points (2, 4, 12, and 18 hpi) have not been accessible before due to the technical challenges associated with liver-stage infections. Our data now provide insights into these early parasite fluxes that may facilitate establishment of infection, transformation, and replication in the liver.
Keywords: P. berghei; Plasmodium; RNA sequencing; liver stage; malaria; transcription.
Copyright © 2020 Toro-Moreno et al.
Figures

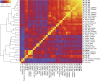
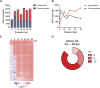


References
-
- World Health Organization. 2019. World malaria report. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
