Targeting CXCR1/2 Does Not Improve Insulin Secretion After Pancreatic Islet Transplantation: A Phase 3, Double-Blind, Randomized, Placebo-Controlled Trial in Type 1 Diabetes
- PMID: 32019854
- PMCID: PMC7876579
- DOI: 10.2337/dc19-1480
Targeting CXCR1/2 Does Not Improve Insulin Secretion After Pancreatic Islet Transplantation: A Phase 3, Double-Blind, Randomized, Placebo-Controlled Trial in Type 1 Diabetes
Abstract
Objective: Reparixin is an inhibitor of CXCR1/2 chemokine receptor shown to be an effective anti-inflammatory adjuvant in a pilot clinical trial in allotransplant recipients.
Research design and methods: A phase 3, multicenter, randomized, double-blind, parallel-assignment study (NCT01817959) was conducted in recipients of islet allotransplants randomized (2:1) to reparixin or placebo in addition to immunosuppression. Primary outcome was the area under the curve (AUC) for C-peptide during the mixed-meal tolerance test at day 75 ± 5 after the first and day 365 ± 14 after the last transplant. Secondary end points included insulin independence and standard measures of glycemic control.
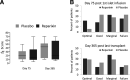
Results: The intention-to-treat analysis did not show a significant difference in C-peptide AUC at both day 75 (27 on reparixin vs. 18 on placebo, P = 0.99) and day 365 (24 on reparixin vs. 15 on placebo, P = 0.71). There was no statistically significant difference between treatment groups at any time point for any secondary variable. Analysis of patient subsets showed a trend for a higher percentage of subjects retaining insulin independence for 1 year after a single islet infusion in patients receiving reparixin as compared with patients receiving placebo (26.7% vs. 0%, P = 0.09) when antithymocyte globulin was used as induction immunosuppression.
Conclusions: In this first double-blind randomized trial, islet transplantation data obtained with reparixin do not support a role of CXCR1/2 inhibition in preventing islet inflammation-mediated damage.
© 2020 by the American Diabetes Association.
Figures

Comment in
-
Improving Clinical Islet Transplantation Outcomes.Diabetes Care. 2020 Apr;43(4):698-700. doi: 10.2337/dci19-0080. Diabetes Care. 2020. PMID: 32198283 No abstract available.
References
-
- Pepper AR, Bruni A, Shapiro AMJ. Clinical islet transplantation: is the future finally now? Curr Opin Organ Transplant 2018;23:428–439 - PubMed
-
- Eich T, Eriksson O, Lundgren T; Nordic Network for Clinical Islet Transplantation . Visualization of early engraftment in clinical islet transplantation by positron-emission tomography. N Engl J Med 2007;356:2754–2755 - PubMed
-
- Malosio ML, Esposito A, Brigatti C, et al. . MR imaging monitoring of iron-labeled pancreatic islets in a small series of patients: islet fate in successful, unsuccessful, and autotransplantation. Cell Transplant 2015;24:2285–2296 - PubMed
-
- Citro A, Cantarelli E, Piemonti L. Anti-inflammatory strategies to enhance islet engraftment and survival. Curr Diab Rep 2013;13:733–744 - PubMed

