Pharmacodynamics of natalizumab extended interval dosing in MS
- PMID: 32019876
- PMCID: PMC7057061
- DOI: 10.1212/NXI.0000000000000672
Pharmacodynamics of natalizumab extended interval dosing in MS
Abstract
Objective: To determine if the concentration and saturation of natalizumab (NTZ) administration at extended interval dosing (EID; every 5-8 weeks) over 18 months is able to be maintained in the range considered adequate to sustain the clinical efficacy of NTZ.
Methods: In a cross-sectional assessment of patients with multiple sclerosis (MS) who received standard interval dosing (every 4 weeks) or EID, serum NTZ concentrations were measured using ELISA, and α4-integrin receptor saturations were analyzed via cytometry, in blood samples obtained at trough timepoints.
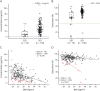
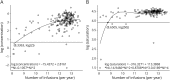
Results: Trough serum concentration was above the "therapeutic" concentration of 2.0 μg/mL in 72% of EID patients. Trough saturation was above the "therapeutic" 50% threshold in 79% of EID-treated patients. Our model predicted that at least 9 NTZ infusions/year are required to maintain adequate trough saturation and concentration levels. Higher body mass index (BMI) was a predictor of suboptimal trough saturation on EID NTZ.
Conclusions: Trough α4-integrin receptor saturation >50% correlated with high clinical efficacy of NTZ in previous studies. A continual treatment with EID maintains receptor saturation and concentration that are in the "therapeutic range" for most patients. This finding provides biological plausibility for the clinical efficacy of NTZ EID. Patients with higher BMI may require closer clinical and MRI follow-up.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
-
- Wipfler P, Harrer A, Pilz G, et al. . Natalizumab saturation: biomarker for individual treatment holiday after natalizumab withdrawal? Acta Neurol Scand 2014;129:e12–e15. - PubMed
-
- Rudick R, Sandrock A. Natalizumab: α4-integrin antagonist selective adhesion molecule inhibitors for MS. Expert Rev Neurother 2004;4:571–580. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
