Long non-coding RNA NNT-AS1 promotes cholangiocarcinoma cells proliferation and epithelial-to-mesenchymal transition through down-regulating miR-203
- PMID: 32019904
- PMCID: PMC7041725
- DOI: 10.18632/aging.102747
Long non-coding RNA NNT-AS1 promotes cholangiocarcinoma cells proliferation and epithelial-to-mesenchymal transition through down-regulating miR-203
Abstract
Background: Cholangiocarcinoma (CCA) is a serious malignant tumor. Long non-coding RNA NNT-AS1 (NNT-AS1) takes crucial roles in several tumors. So, we planned to research the roles and underlying mechanism of NNT-AS1 in CCA.
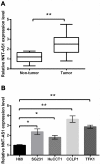
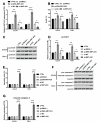
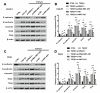
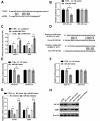
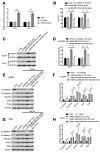
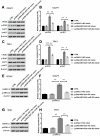
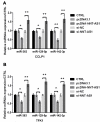
Results: NNT-AS1 overexpression was appeared in CCA tissues and cell lines. Proliferation was promoted by NNT-AS1 overexpression in CCLP1 and TFK1 cells. Besides, NNT-AS1 overexpression reduced E-cadherin level and raised levels of N-cadherin, vimentin, Snail and Slug. However, the opposite trend was occurred by NNT-AS1 knockdown. Further, NNT-AS1 overexpression promoted phosphatidylinositol 3 kinase (PI3K)/AKT and extracellular signal-regulated kinase (ERK)1/2 pathways. MiR-203 was sponged by NNT-AS1 and miR-203 mimic reversed the above promoting effects of NNT-AS1. Additionally, insulin-like growth factor type 1 receptor (IGF1R) and zinc finger E-box binding homeobox 1 (ZEB1) were two potential targets of miR-203.
Conclusion: NNT-AS1 promoted proliferation, EMT and PI3K/AKT and ERK1/2 pathways in CCLP1 and TFK1 cells through down-regulating miR-203.
Methods: CCLP1 and TFK1 cells were co-transfected with pcDNA-NNT-AS1 and miR-203 mimic. Bromodeoxyuridine (BrdU), flow cytometry, quantitative reverse transcription polymerase chain reaction (qRT-PCR) and western blot were employed to detect roles and mechanism of NNT-AS1. Interaction between NNT-AS1 and miR-203 or miR-203 and target genes was examined through luciferase activity experiment.
Keywords: cholangiocarcinoma; epithelial-to-mesenchymal transition; long non-coding RNA NNT-AS1; miR-203; proliferation.
Conflict of interest statement
Figures
Similar articles
-
Upregulation of long non-coding RNA NNT-AS1 promotes osteosarcoma progression by inhibiting the tumor suppressive miR-320a.Cancer Biol Ther. 2019;20(4):413-422. doi: 10.1080/15384047.2018.1538612. Epub 2018 Nov 29. Cancer Biol Ther. 2019. PMID: 30489194 Free PMC article.
-
AR-induced ZEB1-AS1 represents poor prognosis in cholangiocarcinoma and facilitates tumor stemness, proliferation and invasion through mediating miR-133b/HOXB8.Aging (Albany NY). 2020 Jan 24;12(2):1237-1255. doi: 10.18632/aging.102680. Epub 2020 Jan 24. Aging (Albany NY). 2020. PMID: 31978895 Free PMC article.
-
Downregulation of lncRNA ZEB1-AS1 Represses Cell Proliferation, Migration, and Invasion Through Mediating PI3K/AKT/mTOR Signaling by miR-342-3p/CUL4B Axis in Prostate Cancer.Cancer Biother Radiopharm. 2020 Nov;35(9):661-672. doi: 10.1089/cbr.2019.3123. Epub 2020 Apr 9. Cancer Biother Radiopharm. 2020. PMID: 32275162
-
A review on the role of FOXD2-AS1 in human disorders.Pathol Res Pract. 2024 Feb;254:155101. doi: 10.1016/j.prp.2024.155101. Epub 2024 Jan 9. Pathol Res Pract. 2024. PMID: 38211387 Review.
-
The Role of microRNAs in Cholangiocarcinoma.Int J Mol Sci. 2021 Jul 16;22(14):7627. doi: 10.3390/ijms22147627. Int J Mol Sci. 2021. PMID: 34299246 Free PMC article. Review.
Cited by
-
LncRNA-NNT-AS1 contributes to the progression of glioma by miR-582-5p/EZH2 axis.Cytotechnology. 2021 Jun;73(3):473-482. doi: 10.1007/s10616-021-00471-6. Epub 2021 Apr 28. Cytotechnology. 2021. PMID: 34149178 Free PMC article.
-
The Role of Long Non-Coding RNA NNT-AS1 in Neoplastic Disease.Cancers (Basel). 2020 Oct 23;12(11):3086. doi: 10.3390/cancers12113086. Cancers (Basel). 2020. PMID: 33113895 Free PMC article. Review.
-
Overexpression of NNT-AS1 Activates TGF-β Signaling to Decrease Tumor CD4 Lymphocyte Infiltration in Hepatocellular Carcinoma.Biomed Res Int. 2020 Dec 23;2020:8216541. doi: 10.1155/2020/8216541. eCollection 2020. Biomed Res Int. 2020. PMID: 33426064 Free PMC article.
-
Emerging roles of long noncoding RNAs in cholangiocarcinoma: Advances and challenges.Cancer Commun (Lond). 2020 Dec;40(12):655-680. doi: 10.1002/cac2.12109. Epub 2020 Nov 3. Cancer Commun (Lond). 2020. PMID: 33142045 Free PMC article. Review.
-
Characterization of LncRNA SNHG22 as a protector of NKIRAS2 through miR-4492 binding in osteosarcoma.Aging (Albany NY). 2020 Sep 20;12(18):18571-18587. doi: 10.18632/aging.103849. Epub 2020 Sep 20. Aging (Albany NY). 2020. PMID: 32950969 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

