Abundance and diversity of resistomes differ between healthy human oral cavities and gut
- PMID: 32019923
- PMCID: PMC7000725
- DOI: 10.1038/s41467-020-14422-w
Abundance and diversity of resistomes differ between healthy human oral cavities and gut
Abstract
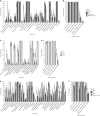
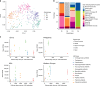
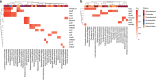
The global threat of antimicrobial resistance has driven the use of high-throughput sequencing techniques to monitor the profile of resistance genes, known as the resistome, in microbial populations. The human oral cavity contains a poorly explored reservoir of these genes. Here we analyse and compare the resistome profiles of 788 oral cavities worldwide with paired stool metagenomes. We find country and body site-specific differences in the prevalence of antimicrobial resistance genes, classes and mechanisms in oral and stool samples. Within individuals, the highest abundances of antimicrobial resistance genes are found in the oral cavity, but the oral cavity contains a lower diversity of resistance genes compared to the gut. Additionally, co-occurrence analysis shows contrasting ARG-species associations between saliva and stool samples. Maintenance and persistence of antimicrobial resistance is likely to vary across different body sites. Thus, we highlight the importance of characterising the resistome across body sites to uncover the antimicrobial resistance potential in the human body.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- World Health Organisation. Antimicrobial Resistance: Global Report on Surveillance. (WHO Press, World Health Organisation, Geneva, 2014). https://eur03.safelinks.protection.outlook.com.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

