Porphyromonas gingivalis triggers the shedding of inflammatory endothelial microvesicles that act as autocrine effectors of endothelial dysfunction
- PMID: 32019950
- PMCID: PMC7000667
- DOI: 10.1038/s41598-020-58374-z
Porphyromonas gingivalis triggers the shedding of inflammatory endothelial microvesicles that act as autocrine effectors of endothelial dysfunction
Abstract
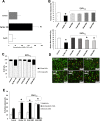
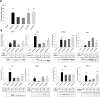
A link between periodontitis and atherothrombosis has been highlighted. The aim of this study was to determine the influence of Porphyromonas gingivalis on endothelial microvesicles (EMVPg) shedding and their contribution to endothelial inflammation. Endothelial cells (EC) were infected with P. gingivalis (MOI = 100) for 24 h. EMVPg were isolated and their concentration was evaluated by prothrombinase assay. EMVPg were significantly increased in comparison with EMVCtrl shedded by unstimulated cells. While EMVCtrl from untreated EC had no effect, whereas, the proportion of apoptotic EC was increased by 30 nM EMVPg and viability was decreased down to 25%, a value elicited by P. gingivalis alone. Moreover, high concentration of EMVPg (30 nM) induced a pro-inflammatory and pro-oxidative cell response including up-regulation of TNF-α, IL-6 and IL-8 as well as an altered expression of iNOS and eNOS at both mRNA and protein level. An increase of VCAM-1 and ICAM-1 mRNA expression (4.5 folds and 3 folds respectively (p < 0.05 vs untreated) was also observed after EMVPg (30 nM) stimulation whereas P. gingivalis infection was less effective, suggesting a specific triggering by EMVPg. Kinasome analysis demonstrated the specific effect induced by EMVPg on main pro-inflammatory pathways including JNK/AKT and STAT. EMVPg are effective pro-inflammatory effectors that may have detrimental effect on vascular homeostasis and should be considered as potential autocrine and paracrine effectors involved in the link between periodontitis and atherothrombosis.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

