A New Function for Perivascular Adipose Tissue (PVAT): Assistance of Arterial Stress Relaxation
- PMID: 32019956
- PMCID: PMC7000722
- DOI: 10.1038/s41598-020-58368-x
A New Function for Perivascular Adipose Tissue (PVAT): Assistance of Arterial Stress Relaxation
Abstract
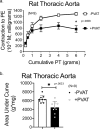
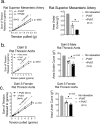
In health, PVAT secretes anti-contractile factors that relax the underlying artery. PVAT's contributions to vascular function include more than production of vasoactive substances. We hypothesized that PVAT benefits the artery by assisting the function of stress (-induced) relaxation. Thoracic aorta rings from Sprague Dawley rats were mounted in isolated tissue baths with (+) and without (-) PVAT. A cumulative length tension (0-6 grams) was generated. The tension to which the tissue stress relaxed over 30 minutes was recorded; the tension lost was stress relaxation. The presence of PVAT increased the amount of stress relaxation (final tension in mgs; aortic ring -PVAT = 4578 ± 190; aortic ring + PVAT = 2730 ± 274, p < 0.05). PVAT left attached but not encompassing the aorta provided no benefit in cumulative stress relaxation (aortic ring +/- PVAT = 4122 ± 176; p > 0.05 vs -PVAT). A PVAT ring separated from the aorta demonstrated more profound stress relaxation than did the aortic ring itself. Finally, PVAT-assisted stress relaxation was observed in an artery with white fat (superior mesenteric artery) and in aorta from both male and female of another rat strain, the Dahl S rat. Knowledge of this new PVAT function supports PVAT as an essential player in vascular health.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin. Exp. Hypertens. A. 1991;13:277–296. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

