Rac1 is a downstream effector of PKCα in structural synaptic plasticity
- PMID: 32019972
- PMCID: PMC7000694
- DOI: 10.1038/s41598-020-58610-6
Rac1 is a downstream effector of PKCα in structural synaptic plasticity
Abstract
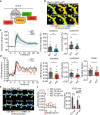
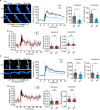
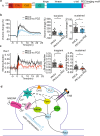
Structural and functional plasticity of dendritic spines is the basis of animal learning. The rapid remodeling of actin cytoskeleton is associated with spine enlargement and shrinkage, which are essential for structural plasticity. The calcium-dependent protein kinase C isoform, PKCα, has been suggested to be critical for this actin-dependent plasticity. However, mechanisms linking PKCα and structural plasticity of spines are unknown. Here, we examine the spatiotemporal activation of actin regulators, including small GTPases Rac1, Cdc42 and Ras, in the presence or absence of PKCα during single-spine structural plasticity. Removal of PKCα expression in the postsynapse attenuated Rac1 activation during structural plasticity without affecting Ras or Cdc42 activity. Moreover, disruption of a PDZ binding domain within PKCα led to impaired Rac1 activation and deficits in structural spine remodeling. These results demonstrate that PKCα positively regulates the activation of Rac1 during structural plasticity.
Conflict of interest statement
Ryohei Yasuda is a founder and a share holder of Florida Lifetime Imaging LLC, a company that helps people set up FLIM.
Figures
Similar articles
-
PI3K couples long-term synaptic potentiation with cofilin recruitment and actin polymerization in dendritic spines via its regulatory subunit p85α.Cell Mol Life Sci. 2024 Aug 19;81(1):358. doi: 10.1007/s00018-024-05394-x. Cell Mol Life Sci. 2024. PMID: 39158722 Free PMC article.
-
Plasticity of dendritic spines: Molecular function and dysfunction in neurodevelopmental disorders.Psychiatry Clin Neurosci. 2019 Sep;73(9):541-550. doi: 10.1111/pcn.12899. Epub 2019 Jul 8. Psychiatry Clin Neurosci. 2019. PMID: 31215705 Review.
-
CD44: a novel synaptic cell adhesion molecule regulating structural and functional plasticity of dendritic spines.Mol Biol Cell. 2016 Dec 15;27(25):4055-4066. doi: 10.1091/mbc.E16-06-0423. Epub 2016 Oct 19. Mol Biol Cell. 2016. PMID: 27798233 Free PMC article.
-
Arhgap22 Disruption Leads to RAC1 Hyperactivity Affecting Hippocampal Glutamatergic Synapses and Cognition in Mice.Mol Neurobiol. 2021 Dec;58(12):6092-6110. doi: 10.1007/s12035-021-02502-x. Epub 2021 Aug 28. Mol Neurobiol. 2021. PMID: 34455539 Free PMC article.
-
Regulation of the actin cytoskeleton in dendritic spines.Adv Exp Med Biol. 2012;970:81-95. doi: 10.1007/978-3-7091-0932-8_4. Adv Exp Med Biol. 2012. PMID: 22351052 Free PMC article. Review.
Cited by
-
Palmitoylated Proteins in Dendritic Spine Remodeling.Front Synaptic Neurosci. 2020 Jun 16;12:22. doi: 10.3389/fnsyn.2020.00022. eCollection 2020. Front Synaptic Neurosci. 2020. PMID: 32655390 Free PMC article.
-
S-Palmitoylation of Synaptic Proteins as a Novel Mechanism Underlying Sex-Dependent Differences in Neuronal Plasticity.Int J Mol Sci. 2021 Jun 10;22(12):6253. doi: 10.3390/ijms22126253. Int J Mol Sci. 2021. PMID: 34200797 Free PMC article.
-
BDNF signaling requires Matrix Metalloproteinase-9 during structural synaptic plasticity.bioRxiv [Preprint]. 2024 Jan 30:2023.12.08.569797. doi: 10.1101/2023.12.08.569797. bioRxiv. 2024. PMID: 38106209 Free PMC article. Preprint.
-
Presynaptic Rac1 in the hippocampus selectively regulates working memory.Elife. 2024 Jul 24;13:RP97289. doi: 10.7554/eLife.97289. Elife. 2024. PMID: 39046788 Free PMC article.
-
B cell receptor-induced IL-10 production from neonatal mouse CD19+CD43- cells depends on STAT5-mediated IL-6 secretion.Elife. 2023 Feb 3;12:e83561. doi: 10.7554/eLife.83561. Elife. 2023. PMID: 36735294 Free PMC article.
References
-
- Hayashi-Takagi Akiko, Yagishita Sho, Nakamura Mayumi, Shirai Fukutoshi, Wu Yi I., Loshbaugh Amanda L., Kuhlman Brian, Hahn Klaus M., Kasai Haruo. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature. 2015;525(7569):333–338. doi: 10.1038/nature15257. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

