Ferumoxytol-enhanced three-dimensional magnetic resonance imaging of carotid atheroma- a feasibility and temporal dependence study
- PMID: 32020031
- PMCID: PMC7000763
- DOI: 10.1038/s41598-020-58708-x
Ferumoxytol-enhanced three-dimensional magnetic resonance imaging of carotid atheroma- a feasibility and temporal dependence study
Abstract
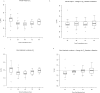
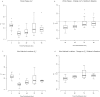
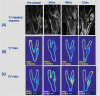
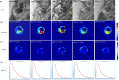
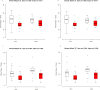
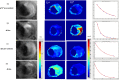
Ferumoxytol is an ultrasmall super paramagnetic particles of iron oxide (USPIO) agent recently used for magnetic resonance (MR) vascular imaging. Other USPIOs have been previously used for assessing inflammation within atheroma. We aim to assess feasibility of ferumoxytol in imaging carotid atheroma (with histological assessment); and the optimum MR imaging time to detect maximum quantitative signal change post-ferumoxytol infusion. Ten patients with carotid artery disease underwent high-resolution MR imaging of their carotid arteries on a 1.5 T MR system. MR imaging was performed before and at 24, 48, 72 and 96 hrs post ferumoxytol infusion. Optimal ferumoxytol uptake time was evaluated by quantitative relaxometry maps indicating the difference in T2* (ΔT2*) and T2 (ΔT2) between baseline and post-Ferumoxytol MR imaging using 3D DANTE MEFGRE qT2*w and iMSDE black-blood qT2w sequences respectively. 20 patients in total (10 symptomatic and 10 with asymptomatic carotid artery disease) had ferumoxytol-enhanced MR imaging at the optimal imaging window. 69 carotid MR imaging studies were completed. Ferumoxytol uptake (determined by a decrease in ΔT2* and ΔT2) was identified in all carotid plaques (symptomatic and asymptomatic). Maximum quantitative decrease in ΔT2* (10.4 [3.5-16.2] ms, p < 0.001) and ΔT2 (13.4 [6.2-18.9] ms; p = 0.001) was found on carotid MR imaging at 48 hrs following the ferumoxytol infusion. Ferumoxytol uptake by carotid plaques was assessed by histopathological analysis of excised atheroma. Ferumoxytol-enhanced MR imaging using quantitative 3D MR pulse sequences allows assessment of inflammation within carotid atheroma in symptomatic and asymptomatic patients. The optimum MR imaging time for carotid atheroma is 48 hrs after its administration.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

