Efficacy of Vildagliptin Added to Continuous Subcutaneous Insulin Infusion (CSII) in Hospitalized Patients with Type 2 Diabetes
- PMID: 32020552
- PMCID: PMC7048888
- DOI: 10.1007/s13300-020-00758-5
Efficacy of Vildagliptin Added to Continuous Subcutaneous Insulin Infusion (CSII) in Hospitalized Patients with Type 2 Diabetes
Abstract
Introduction: The aim of this study was to compare the efficacy of vildagliptin as add-on therapy to short-term continuous subcutaneous insulin infusion (CSII) with CSII monotherapy in hospitalized patients with type 2 diabetes mellitus (T2DM).
Methods: A total of 200 hospitalized patients with inadequately controlled T2DM were randomized into groups, with one group receiving CSII monotherapy (CSII group, n =100) and the other group receiving CSII plus vildagliptin as add-on (CSII + Vig group, n = 100). Of these, 191 completed the 7-day trial (CSII group, n = 99; CSII + Vig group, n = 92) and included in the analysis. The glycemic control and variability of the patients were measured using all-day capillary blood glucose (BG) monitoring. Weight and fasting C-peptide levels were evaluated before and after the interventions.
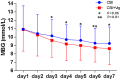
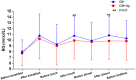
Results: Mean BG concentrations during the whole treatment period were lower and the time to reach target BG was reduced in the CSII + Vig group compared with the CSII group (9.89 ± 3.37 vs. 9.46 ± 3.23 mmol/L, P < 0.01; 129 ± 4 vs. 94 ± 5 h, P < 0.01, respectively). Similarly, the indicators of glycemic variability, namely the standard deviation of BG and the largest amplitude of glycemic excursion, were significantly decreased in the CSII + Vig group compared with the CSII group (2.68 ± 1.05 vs. 2.39 ± 1.00 mmol/L, P < 0.01; 7.19 ± 2.86 vs. 6.23 ± 2.73 mmol/L, P < 0.01, respectively).
Conclusions: Short-term CSII with vildagliptin as add-on therapy may be an optimized treatment for hospitalized patients with T2DM compared with short-term CSII monotherapy.
Keywords: Continuous subcutaneous insulin infusion; Dipeptidyl peptidase-4 inhibitors; Glycemic variability; Hypoglycemia.
Figures

References
-
- Lv WS, Li L, Wen JP. Comparison of a multiple daily insulin injection regimen (glargine or detemir once daily plus prandial insulin aspart) and continuous subcutaneous insulin infusion (aspart) in short-term intensive insulin therapy for poorly controlled type 2 diabetes patients. Int J Endocrinol. 2013;2013:614242. doi: 10.1155/2013/614242. - DOI - PMC - PubMed
-
- Pastores SM. ACP Journal Club. Review: Intensive insulin therapy does not reduce mortality but increases severe hypoglycemia in hospitalized patients. Ann Intern Med. 2011;155(2):JC1–12. - PubMed
LinkOut - more resources
Full Text Sources

