Prolonged-Release (PR) Oxycodone/Naloxone Improves Bowel Function Compared with Oxycodone PR and Provides Effective Analgesia in Chinese Patients with Non-malignant Pain: A Randomized, Double-Blind Trial
- PMID: 32020565
- PMCID: PMC7089730
- DOI: 10.1007/s12325-020-01244-x
Prolonged-Release (PR) Oxycodone/Naloxone Improves Bowel Function Compared with Oxycodone PR and Provides Effective Analgesia in Chinese Patients with Non-malignant Pain: A Randomized, Double-Blind Trial
Abstract
Introduction: Prolonged-release oxycodone/naloxone (OXN PR), combining an opioid analgesic with selective blockade of enteric µ-opioid receptors, provided effective analgesia and improved bowel function in patients with moderate-to-severe pain and opioid-induced constipation in clinical trials predominantly conducted in Western countries. This double-blind randomized controlled trial investigated OXN PR (N = 116) versus prolonged-release oxycodone (OXY PR, N = 115) for 8 weeks at doses up to 50 mg/day in patients with moderate-to-severe, chronic, non-malignant musculoskeletal pain and opioid-induced constipation recruited in China.
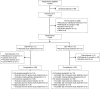
Methods: A total of 234 patients at least 18 years of age with non-malignant musculoskeletal pain for more than 4 weeks that was moderate-to-severe in intensity and required round-the-clock opioid therapy were randomized (1:1) to OXN PR or OXY PR. The primary endpoint was bowel function using the Bowel Function Index (BFI). Secondary endpoints included safety, Brief Pain Inventory-Short Form (BPI-SF), use of analgesic and laxative rescue medication, and health-related quality of life (EQ-5D).
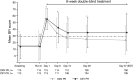
Results: While BFI scores were comparable at baseline, at week 8 improvements were greater with OXN PR vs OXY PR (least squares mean [LSM] difference (95% CI) - 9.1 (- 14.0, - 4.2); P < 0.001. From weeks 2 to 8, mean BFI scores were in the range of normal bowel function (≤ 28.8) with OXN PR but were in the range of constipation (> 28.8) at all timepoints with OXY PR. Analgesia with OXN PR was similar and non-inferior to OXY PR on the basis of modified BPI-SF average 24-h pain scores at week 8: LSM difference (95% CI) - 0.3 (- 0.5, - 0.1); P < 0.001. The most frequent treatment-related AEs were nausea (OXN PR 5% vs OXY PR 6%) and dizziness (4% vs 4%).
Conclusion: OXN PR provided clinically meaningful improvements in bowel function and effective analgesia in Chinese patients with moderate-to-severe musculoskeletal pain and pre-existing opioid-induced constipation.
Trial registration: ClinicalTrials.gov, identifier NCT01918098.
Keywords: Bowel function index; China; Musculoskeletal pain; Opioid-induced constipation; Pain; Prolonged-release oxycodone/naloxone.
Figures

References
-
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. - PubMed
-
- Jackson T, Chen H, Iezzi T, Yee M, Chen F. Prevalence and correlates of chronic pain in a random population study of adults in Chongqing, China. Clin J Pain. 2014;30(4):346–352. - PubMed
-
- Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11(11):1230–1239. - PubMed
-
- Reid KJ, Harker J, Bala MM, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin. 2011;27(2):449–462. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

