Success and efficiency of cell seeding in Avian Tendon Xenografts - A promising alternative for tendon and ligament reconstruction
- PMID: 32021023
- PMCID: PMC6994744
- DOI: 10.1016/j.jor.2019.09.010
Success and efficiency of cell seeding in Avian Tendon Xenografts - A promising alternative for tendon and ligament reconstruction
Abstract
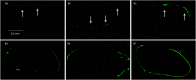
Decellularized tendon xenografts offer a promising alternative for reconstruction by using ubiquitously available material. This study compares static and centrifugal seeding of avian tendon scaffolds with NIH 3T3 fibroblasts. Incorporation of viable cells was achievable with both techniques, represented by DNA content. Proliferation rate and viability assay showed neither damage by centrifugal force nor superiority of the technique. Cell proliferation after 10 days of culture demonstrated that the scaffold did not hinder 3-D culturing. Confocal laser microscopy revealed structural details as formation of focal adhesions, to provide deeper insight into the process of cell attachment and growth in xenografts.
Keywords: Cell adhesion; Cell seeding efficiency; Confocal laser microscopy; Tendon scaffold; Tissue engineering.
© 2019 Professor P K Surendran Memorial Education Foundation. Published by Elsevier B.V. All rights reserved.
Figures






Similar articles
-
Potential of centrifugal seeding method in improving cells distribution and proliferation on demineralized cancellous bone scaffolds for tissue-engineered meniscus.ACS Appl Mater Interfaces. 2015 Jul 22;7(28):15294-302. doi: 10.1021/acsami.5b03129. Epub 2015 Jul 13. ACS Appl Mater Interfaces. 2015. PMID: 26102091
-
Effective cell-seeding technique using magnetite nanoparticles and magnetic force onto decellularized blood vessels for vascular tissue engineering.J Biosci Bioeng. 2007 May;103(5):472-8. doi: 10.1263/jbb.103.472. J Biosci Bioeng. 2007. PMID: 17609164
-
Fabrication and characterization of a decellularized bovine tendon sheet for tendon reconstruction.J Biomed Mater Res A. 2017 Aug;105(8):2299-2311. doi: 10.1002/jbm.a.36083. Epub 2017 May 30. J Biomed Mater Res A. 2017. PMID: 28380688
-
[Experimental study on co-culture of human fibroblasts on decellularized Achilles tendon].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013 Jul;27(7):805-9. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013. PMID: 24063167 Chinese.
-
[Research progress of cell-scaffold complex in tendon tissue engineering].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013 Apr;27(4):481-5. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013. PMID: 23757879 Review. Chinese.
Cited by
-
Current Progress in Vascular Engineering and Its Clinical Applications.Cells. 2022 Jan 31;11(3):493. doi: 10.3390/cells11030493. Cells. 2022. PMID: 35159302 Free PMC article. Review.
References
-
- Butler D.L., Juncosa-Melvin N., Boivin G.P. Functional tissue engineering for tendon repair: a multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. Jan 2008;26(1):1–9. - PubMed
-
- Poehling G.G., Curl W.W., Lee C.A. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. Jul 2005;21(7):774–785. - PubMed
-
- Whitlock P.W., Smith T.L., Poehling G.G., Shilt J.S., Van Dyke M. A naturally derived, cytocompatible, and architecturally optimized scaffold for tendon and ligament regeneration. Biomaterials. Oct 2007;28(29):4321–4329. - PubMed
-
- James R., Kesturu G., Balian G., Chhabra A.B. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg. Jan 2008;33(1):102–112. - PubMed
LinkOut - more resources
Full Text Sources

