RGFP966 Suppresses Tumor Growth and Migration Through Inhibition of EGFR Expression in Hepatocellular Carcinoma Cells in vitro
- PMID: 32021097
- PMCID: PMC6959505
- DOI: 10.2147/DDDT.S234871
RGFP966 Suppresses Tumor Growth and Migration Through Inhibition of EGFR Expression in Hepatocellular Carcinoma Cells in vitro
Abstract
Purpose: Histone deacetylase 3 (HDAC3) has been suggested to play a role in hepatocellular carcinoma (HCC). In the present report, we aimed to identify the effects of RGFP966, a specific HDAC3 inhibitor, on the cell proliferation and migration of HCC cell lines.
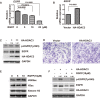
Methods: Human HCC cell lines, which were identified using short tandem repeat (STR) DNA profiling analysis, were used in this report. Cell proliferation assay was used to identify the growth viability of cells. Wound healing and transwell assay were used to identify the migration ability of cells. Further, a human phospho-receptor tyrosine kinases array kit was used to screen out RGFP966 effects on key receptor tyrosine kinases. Then, the mRNA expression was quantified by real-time PCR, and protein expression was identified by Western blot immunoassay.
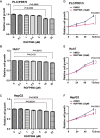
Results: We found that RGFP966 inhibited both proliferation and migration of HCC cells. Further, RGFP966 represses the expression and phosphorylation levels of epidermal growth factor receptor (EGFR) in HCC cells. Moreover, HDAC3 is involved in the inhibition of EGFR by RGFP966. Overall, we elucidated an inhibitive function of RGFP966 in HCC progression.
Conclusion: RGFP966 inhibits EGFR signaling pathway and suppresses proliferation and migration of HCC cells.
Keywords: EGFR; HDAC3; RGFP966; hepatocellular carcinoma.
© 2020 Yu et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Choline Kinase α Mediates Interactions Between the Epidermal Growth Factor Receptor and Mechanistic Target of Rapamycin Complex 2 in Hepatocellular Carcinoma Cells to Promote Drug Resistance and Xenograft Tumor Progression.Gastroenterology. 2017 Apr;152(5):1187-1202. doi: 10.1053/j.gastro.2016.12.033. Epub 2017 Jan 5. Gastroenterology. 2017. PMID: 28065789 Free PMC article.
-
Discovery of CAPE derivatives as dual EGFR and CSK inhibitors with anticancer activity in a murine model of hepatocellular carcinoma.Bioorg Chem. 2021 Feb;107:104536. doi: 10.1016/j.bioorg.2020.104536. Epub 2020 Dec 10. Bioorg Chem. 2021. PMID: 33342565
-
The role of EGF-EGFR signalling pathway in hepatocellular carcinoma inflammatory microenvironment.J Cell Mol Med. 2014 Feb;18(2):218-30. doi: 10.1111/jcmm.12153. Epub 2013 Nov 25. J Cell Mol Med. 2014. PMID: 24268047 Free PMC article.
-
Tunicamycin inhibits cell proliferation and migration in hepatocellular carcinoma through suppression of CD44s and the ERK1/2 pathway.Cancer Sci. 2018 Apr;109(4):1088-1100. doi: 10.1111/cas.13518. Epub 2018 Feb 26. Cancer Sci. 2018. PMID: 29377347 Free PMC article.
-
Treatment with a New Barbituric Acid Derivative Exerts Antiproliferative and Antimigratory Effects against Sorafenib Resistance in Hepatocellular Carcinoma.Molecules. 2020 Jun 20;25(12):2856. doi: 10.3390/molecules25122856. Molecules. 2020. PMID: 32575795 Free PMC article.
Cited by
-
A narrative review of epigenetic marker in H3K27ac and its emerging potential as a therapeutic target in cancer.Epigenomics. 2025 Mar;17(4):263-279. doi: 10.1080/17501911.2025.2460900. Epub 2025 Feb 21. Epigenomics. 2025. PMID: 39981972 Review.
-
HDAC3: A Multifaceted Modulator in Immunotherapy Sensitization.Vaccines (Basel). 2025 Feb 13;13(2):182. doi: 10.3390/vaccines13020182. Vaccines (Basel). 2025. PMID: 40006729 Free PMC article. Review.
-
Super-enhancers complexes zoom in transcription in cancer.J Exp Clin Cancer Res. 2023 Jul 28;42(1):183. doi: 10.1186/s13046-023-02763-5. J Exp Clin Cancer Res. 2023. PMID: 37501079 Free PMC article. Review.
-
Molecular targets of Yangyin Fuzheng Jiedu Prescription in the treatment of hepatocellular carcinoma based on network pharmacology analysis.Cancer Cell Int. 2020 Nov 9;20(1):540. doi: 10.1186/s12935-020-01596-y. Cancer Cell Int. 2020. PMID: 33292207 Free PMC article.
-
HDAC3 inhibitors induce drug resistance by promoting IL-17 A production by T cells.Sci Rep. 2024 Dec 30;14(1):31937. doi: 10.1038/s41598-024-83447-8. Sci Rep. 2024. PMID: 39738540 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

