Biomedical Applications of Zeolitic Nanoparticles, with an Emphasis on Medical Interventions
- PMID: 32021185
- PMCID: PMC6983480
- DOI: 10.2147/IJN.S234573
Biomedical Applications of Zeolitic Nanoparticles, with an Emphasis on Medical Interventions
Abstract
The advent of porous materials, in particular zeolitic nanoparticles, has opened up unprecedented putative research avenues in nanomedicine. Zeolites with intracrystal mesopores are low framework density aluminosilicates possessing a regular porous structure along with intricate channels. Their unique physiochemical as well as physiological parameters necessitate a comprehensive overview on their classifications, fabrication platforms, cellular/macromolecular interactions, and eventually their prospective biomedical applications through illustrating the challenges and opportunities in different integrative medical and pharmaceutical fields. More particularly, an update on recent advances in zeolite-accommodated drug delivery and the prevalent challenges regarding these molecular sieves is to be presented. In conclusion, strategies to accelerate the translation of these porous materials from bench to bedside along with common overlooked physiological and pharmacological factors of zeolite nanoparticles are discussed and debated. Furthermore, for zeolite nanoparticles, it is a matter of crucial importance, in terms of biosafety and nanotoxicology, to appreciate the zeolite-bio interface once the zeolite nanoparticles are exposed to the bio-macromolecules in biological media. We specifically shed light on interactions of zeolite nanoparticles with fibrinogen and amyloid beta which had been comprehensively investigated in our recent reports. Given the significance of zeolite nanoparticles' interactions with serum or interstitial proteins conferring them new biological identity, the preliminary approaches for deeper understanding of administration, distribution, metabolism and excretion of zeolite nanoparticles are elucidated.
Keywords: biomedical applications; biosafety; mesoporous; nanostructure; zeolite.
© 2020 Derakhshankhah et al.
Conflict of interest statement
The authors declare they have no competing interests.
Figures

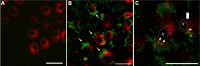
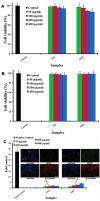


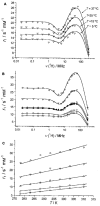
 ), GdNaY-2.3 (
), GdNaY-2.3 ( ), GdNaY-3.6 (
), GdNaY-3.6 ( ), GdNaY-5.0 (
), GdNaY-5.0 ( ) and GdNaY-5.4 (
) and GdNaY-5.4 ( ) and La-2.8-GdNaY-3.3 (
) and La-2.8-GdNaY-3.3 ( ). Reprinted with permission from Platas-Iglesias C, Vander Elst L, Zhou W, et al. Zeolite GdNaY nanoparticles with very high relaxivity for application as contrast agents in magnetic resonance imaging. Chem Eur J. 2002;8(22):5121–5131. Copyright 2002, John Wiley and Sons.
). Reprinted with permission from Platas-Iglesias C, Vander Elst L, Zhou W, et al. Zeolite GdNaY nanoparticles with very high relaxivity for application as contrast agents in magnetic resonance imaging. Chem Eur J. 2002;8(22):5121–5131. Copyright 2002, John Wiley and Sons.
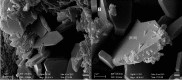


References
-
- Everett DH. Manual of symbols and terminology for physicochemical quantities and units, appendix II: definitions, terminology and symbols in colloid and surface chemistry. Pure Appl Chem. 1972;31(4):577–638. doi:10.1351/pac197231040577 - DOI
-
- Barrer RM. Hydrothermal Chemistry of Zeolites. London: Academic Press; 1982.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

