Doxorubicin-Loaded Carbon Dots Lipid-Coated Calcium Phosphate Nanoparticles for Visual Targeted Delivery and Therapy of Tumor
- PMID: 32021189
- PMCID: PMC6982446
- DOI: 10.2147/IJN.S229154
Doxorubicin-Loaded Carbon Dots Lipid-Coated Calcium Phosphate Nanoparticles for Visual Targeted Delivery and Therapy of Tumor
Abstract
Background: Carbon dots (CDs) have attracted extensive attention in recent years because of their high biocompatibility and unique optical property. But they could not be well applied in the drug delivery system to enable distribution in tumor sites with their low pH sensitivity. They are barriers for drug delivery. CDs as an imaging proper were conjugated with doxorubicin (DOX) lipid-coated calcium phosphate (LCP) nanoparticle, for a pH-sensitive nanocarrier and delivery of the antitumor drugs.
Materials and methods: CDs were prepared by one-step hydrothermal treatment of citric acid and ethylenediamine. The nanoparticles were simply prepared by using microemulsion technology to form calcium phosphate (CaP) core and further coated with cationic lipids.
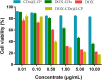
Results: The structure was characterized by FTIR, XRD and TEM. In vitro release study revealed that DOX-CDs@LCP was pH dependent. The cytotoxicity assay demonstrated that it exhibited enhanced efficiency compared to the control group (DOX-CDs), but weaker than free DOX. The cellular uptake revealed that these pH-sensitive nanoparticles could be taken up effectively and deliver DOX into the cytoplasm to reach antitumor effect. The fluorescence imaging indicated that DOX-CDs@LCP mostly distributed in the tumor region due to the enhanced permeability and retention effect (EPR) to reduce its systematical toxicity. Importantly, an antitumor activity study demonstrated that the DOX-CDs@LCP nanoparticles had higher antitumor activity than any other groups and lower toxicity. The results showed that LCP could significantly promote the release in tumor microenvironment due to pH-response. The DOX-CDs could enhance load capacity and reduce drug premature releasing; real-time tracking of efficacy as confocal imaging contrast agent. Thus, DOX-CDs@LCP had antitumor capacity and lower systematic toxicity in tumor therapy.
Conclusion: DOX-CDs@LCP were proven as a promising tumor pH-sensitive and imaging-guided drug delivery system for liver cancer chemotherapy.
Keywords: bioimaging; calcium phosphate photodynamic therapy; carbon dots; pH-sensitive; therapy; tumor targeting.
© 2020 Zhang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures







References
-
- Jeong KH, Woo HS, Kim CJ, et al. Formulation of a modified-release pregabalin tablet using hot-melt coating with glyceryl behenate. Int J Pharm. 2015;495(1):1–8. - PubMed
-
- Fu J, Wang H. Precision diagnosis and treatment of liver cancer in China. Cancer Lett. 2017;412:283. - PubMed
-
- Chamberlain C, Collin SM, Hounsome L, et al. Equity of access to treatment on the cancer drugs fund: a missed opportunity for cancer research? J Cancer Policy. 2015;5:25–30.
-
- Siyuan W, Zhenzhen M, Yuanheng M, et al. Dopamine enhances the response of sunitinib in the treatment of drug-resistant breast cancer: involvement of eradicating cancer stem-like cells. Biochem Pharmacol. 2015;95(2):98–109. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

