Nanocarriers for Stroke Therapy: Advances and Obstacles in Translating Animal Studies
- PMID: 32021190
- PMCID: PMC6982459
- DOI: 10.2147/IJN.S231853
Nanocarriers for Stroke Therapy: Advances and Obstacles in Translating Animal Studies
Abstract
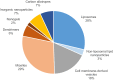
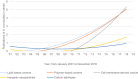
The technology of drug delivery systems (DDS) has expanded into many applications, such as for treating neurological disorders. Nanoparticle DDS offer a unique strategy for targeted transport and improved outcomes of therapeutics. Stroke is likely to benefit from the emergence of this technology though clinical breakthroughs are yet to manifest. This review explores the recent advances in this field and provides insight on the trends, prospects and challenges of translating this technology to clinical application. Carriers of diverse material compositions are presented, with special focus on the surface properties and emphasis on the similarities and inconsistencies among in vivo experimental paradigms. Research attention is scattered among various nanoparticle DDS and various routes of drug administration, which expresses the lack of consistency among studies. Analysis of current literature reveals lipid- and polymer-based DDS as forerunners of DDS for stroke; however, cell membrane-derived vesicles (CMVs) possess the competitive edge due to their innate biocompatibility and superior efficacy. Conversely, inorganic and carbon-based DDS offer different functionalities as well as varied capacity for loading but suffer mainly from poor safety and general lack of investigation in this area. This review supports the existing literature by systematizing presently available data and accounting for the differences in drugs of choice, carrier types, animal models, intervention strategies and outcome parameters.
Keywords: animal model; drug delivery system; nano medicine; nanoparticle; stroke; therapeutics.
© 2020 Alkaff et al.
Conflict of interest statement
The authors declare no competing interest in this work. This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors. Figures of this review were created using Microsoft Office and ChemDraw software, using freely available templates.
Figures



References
-
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics - 2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

