Clinical Utility of Rush Venom Immunotherapy: Current Status
- PMID: 32021308
- PMCID: PMC6954838
- DOI: 10.2147/JAA.S200917
Clinical Utility of Rush Venom Immunotherapy: Current Status
Abstract
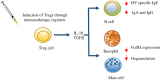
Hymenoptera venom allergy (HVA) is the leading cause of anaphylactic reactions in adults and the second most common cause in children. Venom immunotherapy (VIT) is used to elicit an immune tolerance against hymenoptera venom in allergic patients and is based on the administration of purified venom extracts regularly for defined periods. The protocols of administration include 2 phases: an up-dosing phase that incrementally reaches the final dose resulting in a protective effect, and a maintenance phase in order to obtain the sustained effect. The goal of this review is to detail the efficacy and the safety of the up-dosing phase also named rush. Pathophysiological mechanisms, indications of VIT and technical aspects of up-dosing protocol are also covered.
Keywords: allergy; hymenoptera; immunotherapy.
© 2020 Gruzelle et al.
Conflict of interest statement
LG declares financial and non-financial support from ALK, during the conduct of this work. CM declares non-financial support from ALK. The authors report no other conflicts of interest in this work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

