Extraglycemic Effects of SGLT2 Inhibitors: A Review of the Evidence
- PMID: 32021362
- PMCID: PMC6982447
- DOI: 10.2147/DMSO.S233538
Extraglycemic Effects of SGLT2 Inhibitors: A Review of the Evidence
Abstract
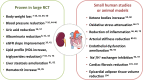
Patients with type 2 diabetes (T2D) are often overweight/obese and affected by arterial hypertension, dyslipidaemia, and have high serum levels of uric acid. Moreover, T2D patient have a higher risk of developing cardiovascular or renal complications, which are leading causes of morbidity and mortality in this population. Sodium-glucose cotransporter-2 inhibitors (SGLT2i) are a new class of glucose-lowering medications that block the reabsorption of glucose in the kidney, thereby increasing urinary glucose excretion, and lowering blood glucose levels. The beneficial effects of SGLT2 inhibition extend beyond glycaemic control, and include improvement in blood pressure, body weight, uric acid concentrations, liver steatosis, oxidative stress, and inflammation. In dedicated cardiovascular outcome trials, SGLT2i treatment was associated with a significant reduction in the rate of cardiovascular events and renal endpoints. In this review, we summarize the evidence for extra-glycemic effects of SGLT2i and the potential mechanisms driving cardiorenal protection exerted by this class of medications.
Keywords: cardiovascular effects; renal effects; review; sodium-glucose cotransporter-2 inhibitors; type 2 diabetes.
© 2020 Bonora et al.
Conflict of interest statement
B.M.B. received lecture or advisory board fees from Astra Zeneca, Eli Lilly, Boehringer Ingelheim, Novo Nordisk, and Novartis, G.P.F. received grant support, lecture fees, or advisory board fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Sanofi, Genzyme, Abbott, Novartis, and Merck Sharp & Dohme. A.A. received research grants, lecture fees, or advisory board fees from Merck Sharp & Dome, AstraZeneca, Novartis, Boehringer Ingelheim, Sanofi, Mediolanum, Janssen, Novo Nordisk, Eli Lilly, Servier, and Takeda. The authors report no other conflicts of interest in this work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources