12-Month clinical outcomes of amphilimus drug eluting stents in an all-comers South-East Asian registry
- PMID: 32021903
- PMCID: PMC6993003
- DOI: 10.1016/j.ijcha.2020.100469
12-Month clinical outcomes of amphilimus drug eluting stents in an all-comers South-East Asian registry
Abstract
Background: Amphilimus-eluting stent (AES) is a novel polymer-free drug eluting stent that combines sirolimus with fatty acid as antiproliferative drug and has shown promising results in percutaneous coronary intervention.We evaluated the clinical safety and efficacy of AES in an all-comers South-East Asian registry.
Methods: Between May 2014 to April 2017, 268 patients (88% male, mean age 60.1 ± 10.8 years) with 291 coronary lesions were treated with AES. The primary endpoint was major adverse cardiac events (MACE) ie a composite of cardiovascular mortality, myocardial infarction (MI) and target lesion revascularization (TLR) at 12-month follow-up.
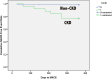
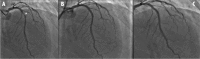
Results: The majority of patients presented with acute coronary syndrome (75%) and 75% had multi-vessel disease on angiography. Diabetes mellitus was present in 123 patients (46%). The most common target vessel for PCI was left anterior descending artery (43%) followed by right coronary artery (36%), left circumflex (10%) and left main (6%).The majority of lesions were type B-C (85%) by ACC/AHA lesion classification. An average of 1.25 ± 0.5 AES were used per patient, with mean AES diameter of 3.1 ± 0.4 mm and average total length of 34.8 ± 19.4 mm.At 12-month follow-up, 4% of patients developed MACE. MACE was mainly driven by cardiovascular mortality (1.5%), MI (2%) and TLR (1.5%). The rate of stent thrombosis was 1.5%.
Conclusion: In a contemporary all-comers South-East Asian registry with high rate of diabetes mellitus, AES was found to be efficacious with a low incidence of MACE observed at 12-month follow-up.
Keywords: Amphilimus; Drug-eluting stent; Outcomes; Percutaneous coronary intervention; South-East Asia.
© 2020 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Carrié D. Advances with polymer-free amphilimus-eluting stents. Minerva Cardioangiol. 2016;64(3):339–353. - PubMed
-
- Carrié D., Berland J., Verheye S. A multicenter randomized trial comparing amphilimus- with paclitaxel-eluting stents in de novo native coronary artery lesions. J. Am. Coll. Cardiol. 2012;59(15):1371–1376. - PubMed
-
- Panoulas V.F., Latib A., Naim C. Clinical outcomes of real-world patients treated with an amphilimus polymer-free stent versus new generation everolimus-eluting stents. Catheter. Cardiovasc. Interv. 2015;86(7):1168–1176. - PubMed
-
- Romaguera R., Gómez-Hospital J.A., Gomez-Lara J. A randomized comparison of reservoir-based polymer-free amphilimus-eluting stents versus everolimus-eluting stents with durable polymer in patients with diabetes mellitus: the RESERVOIR clinical trial. JACC Cardiovasc. Interv. 2016;9(1):42–50. - PubMed
-
- Colombo A., Godino C., Donahue M. One-year clinical outcome of amphilimus polymer-free drug-eluting stent in diabetes mellitus patients: Insight from the ASTUTE registry (AmphilimuS iTalian mUlticenTre rEgistry) Int. J. Cardiol. 2016;1(214):113–120. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

