A Comparison of Psoriasis Severity in Pediatric Patients Treated With Methotrexate vs Biologic Agents
- PMID: 32022846
- PMCID: PMC7042803
- DOI: 10.1001/jamadermatol.2019.4835
A Comparison of Psoriasis Severity in Pediatric Patients Treated With Methotrexate vs Biologic Agents
Abstract
Importance: Few studies have compared the use of methotrexate and biologics, the most commonly used systemic medications for treatment of moderate to severe psoriasis in children.
Objective: To assess the real-world, 6-month reduction in psoriasis severity and long-term drug survival (rate and duration of adherence to a specific drug) of methotrexate vs biologics in plaque psoriasis in children.
Design, setting, and participants: A retrospective medical records review was conducted at 20 European and North American centers. Treatment response was based on site-reported Psoriasis Area and Severity Index (PASI) and/or Physician Global Assessment (PGA) scores at baseline and within the first 6 months of treatment. Participants included all 234 consecutively seen children with moderate to severe psoriasis who received at least 3 months of methotrexate or biologics from December 1, 1990, to September 16, 2014, with sufficient data for analysis. Data analysis was performed from December 14, 2015, to September 1, 2016.
Main outcomes and measures: PASI, with a range from 0 to 72 (highest score indicating severe psoriasis), and/or PGA, with a scale of 0 (clear), 1 (minimal), 2 (mild), 3 (moderate), 4 (severe), and 5 (very severe).
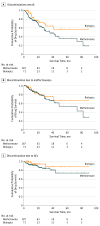
Results: Of 234 pediatric patients (103 boys [44.0%]; 131 girls [56.0%]) treated with methotrexate and/or biologics, 163 patients (69.7%) exclusively received methotrexate, 47 patients (20.1%) exclusively received biologics, and 24 children (10.2%) received methotrexate and biologics sequentially. Of the latter cohort, 23 children were treated initially with methotrexate. Mean (SD) age at initiation was 11.6 (3.7) years for methotrexate and 13.3 (2.9) years for biologics (73.2% for etanercept) (P = .002). Among patients evaluated by a scoring method at 6-month follow-up, 75% or greater improvement in PASI (PASI75) was achieved in 12 of 30 patients (40.0%) receiving methotrexate and 20 of 28 patients (71.4%) receiving biologics, and PGA was clear/almost clear (PGA 0/1) in 41 of 115 patients (35.6%) receiving methotrexate and 18 of 37 patients (48.6%) receiving biologics. Achieving PASI75 and/or PGA 0/1 between baseline and 6 months was more likely with biologics than methotrexate (PASI75: odds ratio [OR], 4.56; 95% CI, 2.02-10.27; P < .001; and PGA 0/1: OR, 2.00; 95% CI, 0.98-4.00; P = .06). Decreased mean PASI and PGA scores were associated with biologics more than with methotrexate (PASI effect, -3.13; 95% CI, -4.33 to -1.94; P < .001; and PGA effect, -0.31; 95% CI, -0.56 to -0.06; P = .02). After 1, 3, and 5 years of use, overall drug survival rates for methotrexate were 77.5%, 50.3%, and 35.9%, and for biologics, the rates were 83.4%, 64.3%, and 57.1%, respectively. Biologics were associated with a better confounder-corrected drug survival than methotrexate (hazard ratio [HR], 2.23; 95% CI, 1.21-4.10; P = .01). Discontinuation owing to lack of response was comparable (HR, 1.64; 95% CI, 0.80-3.36; P = .18).
Conclusions and relevance: Methotrexate and biologics appear to be associated with improvement in pediatric psoriasis, although biologics seem to be associated with greater reduction in psoriasis severity scores and higher drug survival rates than methotrexate in the real-world setting. Additional studies directly comparing these medications should be performed for confirmation.
Conflict of interest statement
Figures
Similar articles
-
Safety of Systemic Agents for the Treatment of Pediatric Psoriasis.JAMA Dermatol. 2017 Nov 1;153(11):1147-1157. doi: 10.1001/jamadermatol.2017.3029. JAMA Dermatol. 2017. PMID: 28903160 Free PMC article.
-
Psoriasis treat to target: defining outcomes in psoriasis using data from a real-world, population-based cohort study (the British Association of Dermatologists Biologics and Immunomodulators Register, BADBIR).Br J Dermatol. 2020 May;182(5):1158-1166. doi: 10.1111/bjd.18333. Epub 2019 Sep 10. Br J Dermatol. 2020. PMID: 31286471 Free PMC article.
-
Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial.Lancet. 2017 Jul 1;390(10089):40-49. doi: 10.1016/S0140-6736(17)31189-3. Epub 2017 May 4. Lancet. 2017. PMID: 28478975 Clinical Trial.
-
Systemic Treatment of Recalcitrant Pediatric Psoriasis: A Case Series and Literature Review.J Drugs Dermatol. 2015 Aug;14(8):881-6. J Drugs Dermatol. 2015. PMID: 26267734 Review.
-
Comparison of Biologics and Oral Treatments for Plaque Psoriasis: A Meta-analysis.JAMA Dermatol. 2020 Mar 1;156(3):258-269. doi: 10.1001/jamadermatol.2019.4029. JAMA Dermatol. 2020. PMID: 32022825 Free PMC article.
Cited by
-
Long-Term Efficacy and Safety of Secukinumab in Children and Adolescents with Moderate-to-Severe Chronic Plaque Psoriasis: Four-Year Results of a Randomized, Phase III, Open-Label Trial.Paediatr Drugs. 2025 Aug 28. doi: 10.1007/s40272-025-00715-4. Online ahead of print. Paediatr Drugs. 2025. PMID: 40874954
-
Efficacy and safety of biological agents for the treatment of pediatric patients with psoriasis: A bayesian analysis of six high-quality randomized controlled trials.Front Immunol. 2022 Aug 19;13:896550. doi: 10.3389/fimmu.2022.896550. eCollection 2022. Front Immunol. 2022. PMID: 36081503 Free PMC article.
-
Therapeutic challenges in managing pediatric psoriasis.Int J Womens Dermatol. 2020 Oct 10;7(3):314-318. doi: 10.1016/j.ijwd.2020.09.012. eCollection 2021 Jun. Int J Womens Dermatol. 2020. PMID: 34222589 Free PMC article. Review.
-
Optimal Management of Plaque Psoriasis in Adolescents: Current Perspectives.Psoriasis (Auckl). 2020 Nov 27;10:45-56. doi: 10.2147/PTT.S222729. eCollection 2020. Psoriasis (Auckl). 2020. PMID: 33274179 Free PMC article. Review.
-
Biologics and Small Molecule Targeted Therapies for Pediatric Alopecia Areata, Psoriasis, Atopic Dermatitis, and Hidradenitis Suppurativa in the US: A Narrative Review.Children (Basel). 2024 Jul 25;11(8):892. doi: 10.3390/children11080892. Children (Basel). 2024. PMID: 39201826 Free PMC article. Review.
References
-
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386(9997):983-994. - PubMed
-
- Eichenfield LF, Paller AS, Tom WL, et al. . Pediatric psoriasis. Pediatr Dermatol. 2018;35(2):170-181. - PubMed
-
- Bronckers IMGJ, Seyger MMB, West DP, et al. ; Psoriasis Investigator Group (PsIG) of the Pediatric Dermatology Research Alliance and the European Working Group on Pediatric Psoriasis (EWGPP) . Safety of systemic agents for the treatment of pediatric psoriasis. JAMA Dermatol. 2017;153():1147-1157. - PMC - PubMed
-
- van Geel MJ, Oostveen AM, Hoppenreijs EP, et al. . Methotrexate in pediatric plaque-type psoriasis. J Dermatolog Treat. 2015;26(5):406-412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

