Hyperoxia Injury in the Developing Lung Is Mediated by Mesenchymal Expression of Wnt5A
- PMID: 32023086
- PMCID: PMC7233334
- DOI: 10.1164/rccm.201908-1513OC
Hyperoxia Injury in the Developing Lung Is Mediated by Mesenchymal Expression of Wnt5A
Abstract
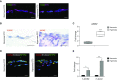
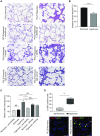
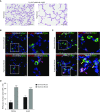
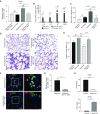
Rationale: Bronchopulmonary dysplasia (BPD) is a leading complication of preterm birth that affects infants born in the saccular stage of lung development at <32 weeks of gestation. Although the mechanisms driving BPD remain uncertain, exposure to hyperoxia is thought to contribute to disease pathogenesis.Objectives: To determine the effects of hyperoxia on epithelial-mesenchymal interactions and to define the mediators of activated Wnt/β-catenin signaling after hyperoxia injury.Methods: Three hyperoxia models were used: A three-dimensional organotypic coculture using primary human lung cells, precision-cut lung slices (PCLS), and a murine in vivo hyperoxia model. Comparisons of normoxia- and hyperoxia-exposed samples were made by real-time quantitative PCR, RNA in situ hybridization, quantitative confocal microscopy, and lung morphometry.Measurements and Main Results: Examination of an array of Wnt ligands in the three-dimensional organotypic coculture revealed increased mesenchymal expression of WNT5A. Inhibition of Wnt5A abrogated the BPD transcriptomic phenotype induced by hyperoxia. In the PCLS model, Wnt5A inhibition improved alveolarization following hyperoxia exposure, and treatment with recombinant Wnt5a reproduced features of the BPD phenotype in PCLS cultured in normoxic conditions. Chemical inhibition of NF-κB with BAY11-7082 reduced Wnt5a expression in the PCLS hyperoxia model and in vivo mouse hyperoxia model, with improved alveolarization in the PCLS model.Conclusions: Increased mesenchymal Wnt5A during saccular-stage hyperoxia injury contributes to the impaired alveolarization and septal thickening observed in BPD. Precise targeting of Wnt5A may represent a potential therapeutic strategy for the treatment of BPD.
Keywords: Wnt; bronchopulmonary dysplasia; hyperoxia; lung injury.
Figures



Comment in
-
The Wnt Signaling Pathway and the Development of Bronchopulmonary Dysplasia.Am J Respir Crit Care Med. 2020 May 15;201(10):1174-1176. doi: 10.1164/rccm.202002-0277ED. Am J Respir Crit Care Med. 2020. PMID: 32101467 Free PMC article. No abstract available.
References
-
- Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. - PubMed
-
- Surate Solaligue DE, Rodríguez-Castillo JA, Ahlbrecht K, Morty RE. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2017;313:L1101–L1153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K22 HL113039/HL/NHLBI NIH HHS/United States
- P01 HL092870/HL/NHLBI NIH HHS/United States
- R01 GM108807/GM/NIGMS NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R01 HL127173/HL/NHLBI NIH HHS/United States
- K08 HL143051/HL/NHLBI NIH HHS/United States
- R01 HL128996/HL/NHLBI NIH HHS/United States
- U01 HL101794/HL/NHLBI NIH HHS/United States
- L40 HL138848/HL/NHLBI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- U01 HL122626/HL/NHLBI NIH HHS/United States
- R01 HL129907/HL/NHLBI NIH HHS/United States
- P01 HL116263/HL/NHLBI NIH HHS/United States
- R01 CA218526/CA/NCI NIH HHS/United States
- R01 HL141380/HL/NHLBI NIH HHS/United States
- K12 HD087023/HD/NICHD NIH HHS/United States
- K08 HL127102/HL/NHLBI NIH HHS/United States
- R35 GM122516/GM/NIGMS NIH HHS/United States
- R01 HL085317/HL/NHLBI NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

