A mechanism for neurofilament transport acceleration through nodes of Ranvier
- PMID: 32023144
- PMCID: PMC7202067
- DOI: 10.1091/mbc.E19-09-0509
A mechanism for neurofilament transport acceleration through nodes of Ranvier
Abstract
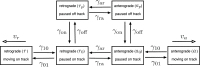
Neurofilaments are abundant space-filling cytoskeletal polymers in axons that are transported along microtubule tracks. Neurofilament transport is accelerated at nodes of Ranvier, where axons are locally constricted. Strikingly, these constrictions are accompanied by sharp decreases in neurofilament number, no decreases in microtubule number, and increases in the packing density of these polymers, which collectively bring nodal neurofilaments closer to their microtubule tracks. We hypothesize that this leads to an increase in the proportion of time that the filaments spend moving and that this can explain the local acceleration. To test this, we developed a stochastic model of neurofilament transport that tracks their number, kinetic state, and proximity to nearby microtubules in space and time. The model assumes that the probability of a neurofilament moving is dependent on its distance from the nearest available microtubule track. Taking into account experimentally reported numbers and densities for neurofilaments and microtubules in nodes and internodes, we show that the model is sufficient to explain the local acceleration of neurofilaments within nodes of Ranvier. This suggests that proximity to microtubule tracks may be a key regulator of neurofilament transport in axons, which has implications for the mechanism of neurofilament accumulation in development and disease.
Figures






Similar articles
-
Local Acceleration of Neurofilament Transport at Nodes of Ranvier.J Neurosci. 2019 Jan 23;39(4):663-677. doi: 10.1523/JNEUROSCI.2272-18.2018. Epub 2018 Dec 12. J Neurosci. 2019. PMID: 30541916 Free PMC article.
-
A possible mechanism for neurofilament slowing down in myelinated axon: Phosphorylation-induced variation of NF kinetics.PLoS One. 2021 Mar 12;16(3):e0247656. doi: 10.1371/journal.pone.0247656. eCollection 2021. PLoS One. 2021. PMID: 33711034 Free PMC article.
-
Phosphorylation-dependent neurofilament epitopes are reduced at the node of Ranvier.J Neurocytol. 1992 Mar;21(3):199-210. doi: 10.1007/BF01194978. J Neurocytol. 1992. PMID: 1373184
-
The effect of myelinating Schwann cells on axons.Muscle Nerve. 2001 Apr;24(4):456-66. doi: 10.1002/mus.1027. Muscle Nerve. 2001. PMID: 11268016 Review.
-
Immunocytochemical investigations of sodium channels along nodal and internodal portions of demyelinated axons.Microsc Res Tech. 1996 Aug 1;34(5):445-51. doi: 10.1002/(SICI)1097-0029(19960801)34:5<445::AID-JEMT4>3.0.CO;2-L. Microsc Res Tech. 1996. PMID: 8837020 Review.
Cited by
-
The node of Ranvier influences the in vivo axonal transport of mitochondria and signaling endosomes.iScience. 2024 Oct 11;27(11):111158. doi: 10.1016/j.isci.2024.111158. eCollection 2024 Nov 15. iScience. 2024. PMID: 39524336 Free PMC article.
-
The role of neurofilament transport in the radial growth of myelinated axons.Mol Biol Cell. 2023 May 15;34(6):ar58. doi: 10.1091/mbc.E22-12-0565. Epub 2023 Feb 22. Mol Biol Cell. 2023. PMID: 36811626 Free PMC article.
-
Caliber of zebrafish touch-sensory axons is dynamic in vivo.bioRxiv [Preprint]. 2025 Jan 24:2024.12.04.626901. doi: 10.1101/2024.12.04.626901. bioRxiv. 2025. Update in: eNeuro. 2025 May 27;12(5):ENEURO.0043-25.2025. doi: 10.1523/ENEURO.0043-25.2025. PMID: 39713467 Free PMC article. Updated. Preprint.
-
Multiscale Mechanobiology in Brain Physiology and Diseases.Front Cell Dev Biol. 2022 Mar 28;10:823857. doi: 10.3389/fcell.2022.823857. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35419366 Free PMC article. Review.
-
Caliber of Rohon-Beard Touch-Sensory Axons Is Dynamic In Vivo.eNeuro. 2025 May 27;12(5):ENEURO.0043-25.2025. doi: 10.1523/ENEURO.0043-25.2025. Print 2025 May. eNeuro. 2025. PMID: 40341239 Free PMC article.
References
-
- Batchelor GK. (1970). Slender-body theory for particles of arbitrary cross-section in Stokes flow. J Fluid Mech , 419–440.
-
- Berthold CH. (1978). Morphology of normal peripheral axons. In: Physiology and Pathobiology of Axons, ed. Waxman SG, New York: Raven Press, 3–63.
-
- Brown A. (2014). Slow axonal transport. In: Reference Module in Biomedical Sciences, ed. Caplan M, Elsevier.
-
- Brown A. (2015). Axonal transport. In: Neuroscience in the 21st Century: From Basic to Clinical, 2nd ed., ed. Pfaff DW, Volkow ND, New York: Springer Publishing, 333–379.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

