Object and place information processing by CA1 hippocampal neurons of C57BL/6J mice
- PMID: 32023149
- PMCID: PMC7099482
- DOI: 10.1152/jn.00278.2019
Object and place information processing by CA1 hippocampal neurons of C57BL/6J mice
Abstract
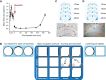
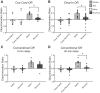
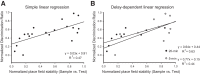
Medial and lateral entorhinal cortices convey spatial/contextual and item/object information to the hippocampus, respectively. Whether the distinct inputs are integrated as one cognitive map by hippocampal neurons to represent location and the objects therein, or whether they remain as parallel outputs, to be integrated in a downstream region, remains unclear. Principal, or complex spike bursting, neurons of hippocampus exhibit location-specific firing, and it is likely that the activity of "place cells" supports spatial memory/navigation in rodents. Consistent with cognitive map theory, the activity of CA1 hippocampal neurons is also critical for nonspatial memory, such as object recognition. However, the degree to which CA1 neuronal activity represents the associations of object-context or object-in-place memory is not well understood. Here, the contributions of mouse CA1 neuronal activity to object recognition memory and the emergence of object-place conjunctive representations were tested using in vivo recordings and functional inactivation. Independent of arena configuration, CA1 place fields were stable throughout testing and object-place representations were not identified in CA1, although the number of fields per cell increased during object sessions, and few object-related firing CA1 neurons (nonplace) were recorded. The results of the inactivation studies confirmed the significant contribution of CA1 neuronal activity to object recognition memory when a delay of 20 min, but not 5 min, was imposed between encoding and retrieval. Together, our results confirm the delay-dependent contribution of the CA1 region to object memory and suggest that object information is processed in parallel with the ongoing spatial mapping function that is a hallmark of hippocampal memory.NEW & NOTEWORTHY We developed variations of the object recognition task to examine the contribution of mouse CA1 neuronal activity to object memory and the degree to which object-context conjunctive representations are formed during object training. Our results indicate that, within the CA1 region, object information is processed in a parallel but delay-dependent manner, with ongoing spatial mapping.
Keywords: CA1; contextual information; memory; muscimol; object recognition.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
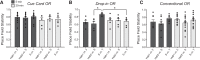


References
-
- Amaral DG, Witter MP. Hippocampal formation. In: The Rat Nervous System, edited by Paxinos G. San Diego, CA: Academic Press Inc, 1995, p. 443–493.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

