Six-Year Follow-up of a Trial of Antenatal Vitamin D for Asthma Reduction
- PMID: 32023372
- PMCID: PMC7444088
- DOI: 10.1056/NEJMoa1906137
Six-Year Follow-up of a Trial of Antenatal Vitamin D for Asthma Reduction
Abstract
Background: We previously reported the results of a trial of prenatal vitamin D supplementation to prevent asthma and recurrent wheeze in young children, which suggested that supplementation provided a protective effect at the age of 3 years. We followed the children through the age of 6 years to determine the course of asthma and recurrent wheeze.
Methods: In this follow-up study, investigators and participants remained unaware of the treatment assignments through the children's sixth birthday. We aimed to determine whether, when maternal levels of 25-hydroxyvitamin D were taken into account, children born to mothers who had received 4400 IU of vitamin D3 per day during pregnancy (vitamin D group) would have a lower incidence of asthma and recurrent wheeze at the age of 6 years than would those born to mothers who had received 400 IU of vitamin D3 per day (control group). Time-to-event methods were used to compare the treatment groups with respect to time to the onset of asthma or recurrent wheeze. Multivariate methods were used to compare longitudinal measures of lung function between the treatment groups.
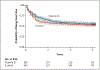
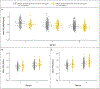
Results: There was no effect of maternal vitamin D supplementation on asthma and recurrent wheeze in either an intention-to-treat analysis or an analysis with stratification according to the maternal 25-hydroxyvitamin D level during pregnancy. There was no effect of prenatal vitamin D supplementation on most of the prespecified secondary outcomes. We found no effects of prenatal supplementation on spirometric indexes. Although there was a very small effect on airway resistance as measured by impulse oscillometry, this finding was of uncertain significance.
Conclusions: Vitamin D supplementation during the prenatal period alone did not influence the 6-year incidence of asthma and recurrent wheeze among children who were at risk for asthma. (Funded by the National Heart, Lung, and Blood Institute; VDAART ClinicalTrials.gov number, NCT00920621.).
Copyright © 2020 Massachusetts Medical Society.
Figures
Comment in
-
Vitamin D Supplementation during Pregnancy and the Prevention of Childhood Asthma.N Engl J Med. 2020 Feb 6;382(6):574-575. doi: 10.1056/NEJMe1915082. N Engl J Med. 2020. PMID: 32023379 No abstract available.
-
Gestational vitamin D supplementation does not reduce asthma incidence in children at 6-year follow-up.Arch Dis Child Educ Pract Ed. 2022 Jun;107(3):231. doi: 10.1136/archdischild-2020-319327. Epub 2020 Sep 10. Arch Dis Child Educ Pract Ed. 2022. PMID: 32912927 No abstract available.
References
-
- Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol 2007; 120: 1031–5. - PubMed
-
- Litonjua AA, Lange NE, Carey VJ, et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp Clin Trials 2014; 38: 37–50. - PMC - PubMed
-
- Wolsk HM, Harshfield BJ, Laranjo N, et al. Vitamin D supplementation in pregnancy, prenatal 25(OH)D levels, race, and subsequent asthma or recurrent wheeze in offspring: secondary analyses from the Vitamin D Antenatal Asthma Reduction Trial. J Allergy Clin Immunol 2017; 140(5): 1423–1429.e5. - PubMed
