Quercetin and Coumarin Inhibit Dipeptidyl Peptidase-IV and Exhibits Antioxidant Properties: In Silico, In Vitro, Ex Vivo
- PMID: 32023875
- PMCID: PMC7072504
- DOI: 10.3390/biom10020207
Quercetin and Coumarin Inhibit Dipeptidyl Peptidase-IV and Exhibits Antioxidant Properties: In Silico, In Vitro, Ex Vivo
Abstract
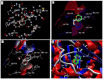
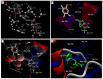
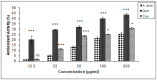
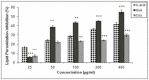
Quercetin and coumarin, two naturally occurring phytochemicals of plant origin, are known to regulate hyperglycemia and oxidative stress. The present study was designed to evaluate the inhibitory activity of quercetin and coumarin on dipeptidyl peptidase-IV (DPP-IV) and their antioxidant potential. DPP-IV inhibition assays were performed, and evaluated IC50 values of diprotin A, quercetin, coumarin, and sitagliptin were found to be 0.653, 4.02, 54.83, and 5.49 nmol/mL, respectively. Furthermore, in silico studies such as the drug-likeliness and docking efficiency of quercetin and coumarin to the DPP-IV protein were performed; the ex vivo antiperoxidative potential of quercetin and coumarin were also evaluated. The results of the present study showed that the DPP-IV inhibitory potential of quercetin was slightly higher than that of sitagliptin. Virtual docking revealed the tight binding of quercetin with DPP-IV protein. Quercetin and coumarin reduced oxidative stress in vitro and ex vivo systems. We report for the first time that both compounds inhibited the DPP-IV along with antioxidant activity and thus may be use as function food ingredients in the prevention of diabetes.
Keywords: antiperoxidative; coumarin; diabetes; dipeptidyl peptidase-IV; hyperglycemia; quercetin.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures



References
-
- Chaudhury A., Duvoor C., Dendi R., Sena V., Kraleti S., Chada A., Ravilla R., Marco A., Shekhawat N.S., Montales M.T. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front. Endocrinol. (Lausanne) 2017;8:6. doi: 10.3389/fendo.2017.00006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

