Human Serum Albumin Aggregation/Fibrillation and its Abilities to Drugs Binding
- PMID: 32023900
- PMCID: PMC7038104
- DOI: 10.3390/molecules25030618
Human Serum Albumin Aggregation/Fibrillation and its Abilities to Drugs Binding
Abstract
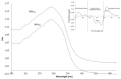
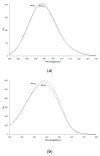
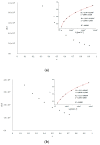
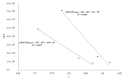
Human serum albumin (HSA) is a protein that transports neutral and acid ligands in the organism. Depending on the environment's pH conditions, HSA can take one of the five isomeric forms that change its conformation. HSA can form aggregates resembling those in vitro formed from amyloid at physiological pH (neutral and acidic). Not surprisingly, the main goal of the research was aggregation/fibrillation of HSA, the study of the physicochemical properties of formed amyloid fibrils using thioflavin T (ThT) and the analysis of ligand binding to aggregated/fibrillated albumin in the presence of dansyl-l-glutamine (dGlu), dansyl-l-proline (dPro), phenylbutazone (Phb) and ketoprofen (Ket). Solutions of human serum albumin, both non-modified and modified, were examined with the use of fluorescence, absorption and circular dichroism (CD) spectroscopy. The experiments conducted allowed observation of changes in the structure of incubated HSA (HSAINC) in relation to nonmodified HSA (HSAFR). The formed aggregates/fibrillation differed in structure from HSA monomers and dimers. Based on CD spectroscopy, previously absent βstructural constructs have been registered. Whereas, using fluorescence spectroscopy, the association constants differing for fresh and incubated HSA solutions in the presence of dansyl-amino acids and markers for binding sites were calculated and allowed observation of the conformational changes in HSA molecule.
Keywords: aggregation/fibrillation; human serum albumin; spectroscopic methods.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures









Similar articles
-
Drug binding properties of glycosylated human serum albumin as measured by fluorescence and circular dichroism.Biol Pharm Bull. 1994 Jan;17(1):16-21. doi: 10.1248/bpb.17.16. Biol Pharm Bull. 1994. PMID: 8148809
-
Biophysical insights into sodium lauroyl sarcosine induced amyloid fibrillation of human serum albumin.Spectrochim Acta A Mol Biomol Spectrosc. 2025 Jul 5;335:125976. doi: 10.1016/j.saa.2025.125976. Epub 2025 Feb 28. Spectrochim Acta A Mol Biomol Spectrosc. 2025. PMID: 40049020
-
Food Additive Hexametaphosphate Promotes Amyloid Formation in Human Serum Albumin: A Molecular Insight.J Mol Recognit. 2025 May;38(3):e70007. doi: 10.1002/jmr.70007. J Mol Recognit. 2025. PMID: 40544348
-
Human Serum Albumin Aggregation and its Modulation Using Nanoparticles: A Review.Protein Pept Lett. 2022;29(1):11-21. doi: 10.2174/0929866528666211125104600. Protein Pept Lett. 2022. PMID: 34823451 Review.
-
Serum Albumin: A Multifaced Enzyme.Int J Mol Sci. 2021 Sep 18;22(18):10086. doi: 10.3390/ijms221810086. Int J Mol Sci. 2021. PMID: 34576249 Free PMC article. Review.
Cited by
-
Isolation and Characterization of Chicken Serum Albumin (Hen Egg Alpha-Livetin, Gal d 5).Foods. 2022 Jun 1;11(11):1637. doi: 10.3390/foods11111637. Foods. 2022. PMID: 35681387 Free PMC article.
-
A General Small-Angle X-ray Scattering-Based Screening Protocol for Studying Physical Stability of Protein Formulations.Pharmaceutics. 2021 Dec 28;14(1):69. doi: 10.3390/pharmaceutics14010069. Pharmaceutics. 2021. PMID: 35056965 Free PMC article.
-
Pre-Formulation Studies: Physicochemical Characteristics and In Vitro Release Kinetics of Insulin from Selected Hydrogels.Pharmaceutics. 2021 Aug 6;13(8):1215. doi: 10.3390/pharmaceutics13081215. Pharmaceutics. 2021. PMID: 34452176 Free PMC article.
-
Changes in the Protein Secondary Structure on the Surface of Silica Nanoparticles with Different Sizes.Langmuir. 2025 Jun 17;41(23):15143-15148. doi: 10.1021/acs.langmuir.5c01606. Epub 2025 Jun 3. Langmuir. 2025. PMID: 40461412 Free PMC article.
-
The Influence of Oxidative Stress on Serum Albumin Structure as a Carrier of Selected Diazaphenothiazine with Potential Anticancer Activity.Pharmaceuticals (Basel). 2021 Mar 23;14(3):285. doi: 10.3390/ph14030285. Pharmaceuticals (Basel). 2021. PMID: 33806875 Free PMC article.
References
-
- Gulam R., Saeyoung N.A. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: A natural cargo. Int. J. Biol. Macromol. 2019;123:979–990. - PubMed
-
- Carter D.C., Ho J.X. Structure of serum albumin. Adv. Protein Chem. 1994;45:153–203. - PubMed
-
- Peters T. Biochemistry, Genetics, And Medical Applications. Academic Press; San Diego, CA, USA: 1995. All About Albumin; pp. 9–19, 228–234.
-
- Sudlow G., Birkett D.J., Wade D.N. Further characterization of specific drug binding sites on human serum albumin. Mol. Pharmacol. 1976;12:1052–1061. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

