Nano-in-Micro Dual Delivery Platform for Chronic Wound Healing Applications
- PMID: 32024165
- PMCID: PMC7074578
- DOI: 10.3390/mi11020158
Nano-in-Micro Dual Delivery Platform for Chronic Wound Healing Applications
Abstract
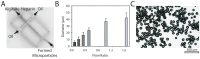
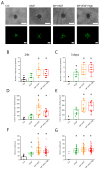
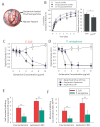
Here, we developed a combinatorial delivery platform for chronic wound healing applications. A microfluidic system was utilized to form a series of biopolymer-based microparticles with enhanced affinity to encapsulate and deliver vascular endothelial growth factor (VEGF). Presence of heparin into the structure can significantly increase the encapsulation efficiency up to 95% and lower the release rate of encapsulated VEGF. Our in vitro results demonstrated that sustained release of VEGF from microparticles can promote capillary network formation and sprouting of endothelial cells in 2D and 3D microenvironments. These engineered microparticles can also encapsulate antibiotic-loaded nanoparticles to offer a dual delivery system able to fight bacterial infection while promoting angiogenesis. We believe this highly tunable drug delivery platform can be used alone or in combination with other wound care products to improve the wound healing process and promote tissue regeneration.
Keywords: angiogenesis; antibacterial properties; chronic wounds; drug delivery; microfluidics; microparticles; nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures