The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation
- PMID: 32024289
- PMCID: PMC7077443
- DOI: 10.3390/polym12020298
The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation
Abstract
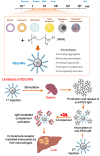
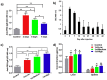
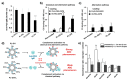
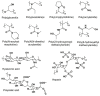
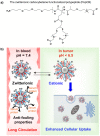
Poly(ethylene glycol) (PEG) is widely used as a gold standard in bioconjugation and nanomedicine to prolong blood circulation time and improve drug efficacy. The conjugation of PEG to proteins, peptides, oligonucleotides (DNA, small interfering RNA (siRNA), microRNA (miRNA)) and nanoparticles is a well-established technique known as PEGylation, with PEGylated products have been using in clinics for the last few decades. However, it is increasingly recognized that treating patients with PEGylated drugs can lead to the formation of antibodies that specifically recognize and bind to PEG (i.e., anti-PEG antibodies). Anti-PEG antibodies are also found in patients who have never been treated with PEGylated drugs but have consumed products containing PEG. Consequently, treating patients who have acquired anti-PEG antibodies with PEGylated drugs results in accelerated blood clearance, low drug efficacy, hypersensitivity, and, in some cases, life-threatening side effects. In this succinct review, we collate recent literature to draw the attention of polymer chemists to the issue of PEG immunogenicity in drug delivery and bioconjugation, thereby highlighting the importance of developing alternative polymers to replace PEG. Several promising yet imperfect alternatives to PEG are also discussed. To achieve asatisfactory alternative, further joint efforts of polymer chemists and scientists in related fields are urgently needed to design, synthesize and evaluate new alternatives to PEG.
Keywords: PEG immunogenicity; PEGylation; anti-PEG antibody; bioconjugation; cancer; drug delivery; nanomedicine; vaccine.
Conflict of interest statement
Authors declare that they have no conflicts of interest.
Figures



References
-
- Alconcel S.N.S., Baas A.S., Maynard H.D. FDA-approved poly(ethylene glycol)–protein conjugate drugs. Polym. Chem. 2011;2:1442–1448. doi: 10.1039/c1py00034a. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

