Role of RUNX Family Transcription Factors in DNA Damage Response
- PMID: 32024352
- PMCID: PMC7057837
- DOI: 10.14348/molcells.2019.0304
Role of RUNX Family Transcription Factors in DNA Damage Response
Abstract
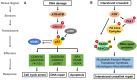
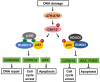
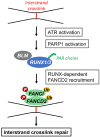
Cells are constantly exposed to endogenous and exogenous stresses that can result in DNA damage. In response, they have evolved complex pathways to maintain genomic integrity. RUNX family transcription factors (RUNX1, RUNX2, and RUNX3 in mammals) are master regulators of development and differentiation, and are frequently dysregulated in cancer. A growing body of research also implicates RUNX proteins as regulators of the DNA damage response, often acting in conjunction with the p53 and Fanconi anemia pathways. In this review, we discuss the functional role and mechanisms involved in RUNX factor mediated response to DNA damage and other cellular stresses. We highlight the impact of these new findings on our understanding of cancer predisposition associated with RUNX factor dysregulation and their implications for designing novel approaches to prevent cancer formation in affected individuals.
Keywords: DNA damage response; Fanconi anemia; RUNX1; RUNX2; RUNX3; cancer; cell cycle arrest; p53; tumor suppressor.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures
References
-
- Akech J., Wixted J.J., Bedard K., van der Deen M., Hussain S., Guise T.A., van Wijnen A.J., Stein J.L., Languino L.R., Altieri D.C., et al. Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene. 2010;29:811–821. doi: 10.1038/onc.2009.389. - DOI - PMC - PubMed
-
- Alcalay M., Meani N., Gelmetti V., Fantozzi A., Fagioli M., Orleth A., Riganelli D., Sebastiani C., Cappelli E., Casciari C., et al. Acute myeloid leukemia fusion proteins deregulate genes involved in stem cell maintenance and DNA repair. J. Clin. Invest. 2003;112:1751–1761. doi: 10.1172/JCI17595. - DOI - PMC - PubMed
-
- Antony-Debré I., Manchev V.T., Balayn N., Bluteau D., Tomowiak C., Legrand C., Langlois T., Bawa O., Tosca L., Tachdjian G., et al. Level of RUNX1 activity is critical for leukemic predisposition but not for thrombocytopenia. Blood. 2015;125:930–940. doi: 10.1182/blood-2014-06-585513. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

