Production of ent-kaurene from lignocellulosic hydrolysate in Rhodosporidium toruloides
- PMID: 32024522
- PMCID: PMC7003354
- DOI: 10.1186/s12934-020-1293-8
Production of ent-kaurene from lignocellulosic hydrolysate in Rhodosporidium toruloides
Abstract
Background: Rhodosporidium toruloides has emerged as a promising host for the production of bioproducts from lignocellulose, in part due to its ability to grow on lignocellulosic feedstocks, tolerate growth inhibitors, and co-utilize sugars and lignin-derived monomers. Ent-kaurene derivatives have a diverse range of potential applications from therapeutics to novel resin-based materials.
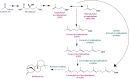
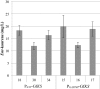
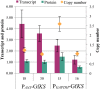
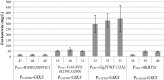
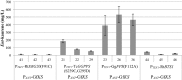
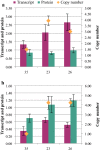
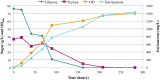
Results: The Design, Build, Test, and Learn (DBTL) approach was employed to engineer production of the non-native diterpene ent-kaurene in R. toruloides. Following expression of kaurene synthase (KS) in R. toruloides in the first DBTL cycle, a key limitation appeared to be the availability of the diterpene precursor, geranylgeranyl diphosphate (GGPP). Further DBTL cycles were carried out to select an optimal GGPP synthase and to balance its expression with KS, requiring two of the strongest promoters in R. toruloides, ANT (adenine nucleotide translocase) and TEF1 (translational elongation factor 1) to drive expression of the KS from Gibberella fujikuroi and a mutant version of an FPP synthase from Gallus gallus that produces GGPP. Scale-up of cultivation in a 2 L bioreactor using a corn stover hydrolysate resulted in an ent-kaurene titer of 1.4 g/L.
Conclusion: This study builds upon previous work demonstrating the potential of R. toruloides as a robust and versatile host for the production of both mono- and sesquiterpenes, and is the first demonstration of the production of a non-native diterpene in this organism.
Keywords: Diterpene; Geranylgeranyl pyrophosphate synthase; Metabolic engineering; Mevalonate pathway; Mutant farnesyl pyrophosphate synthase; Rhodotorula.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Poulter CD, Rilling HC. The prenyl transfer reaction. Enzymic and mechanistic studies of the 1′-4 coupling reaction in the terpene biosynthetic pathway. Acc Chem Res. 1978 doi: 10.1021/ar50128a004. - DOI
-
- Poulter CD. Farnesyl diphosphate synthase. A paradigm for understanding structure and function relationships in E-polyprenyl diphosphate synthases. Phytochem Rev. 2006 doi: 10.1007/s11101-005-4887-1. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

