MR biomarkers predict clinical function in Duchenne muscular dystrophy
- PMID: 32024675
- PMCID: PMC7238941
- DOI: 10.1212/WNL.0000000000009012
MR biomarkers predict clinical function in Duchenne muscular dystrophy
Abstract
Objective: To investigate the potential of lower extremity magnetic resonance (MR) biomarkers to serve as endpoints in clinical trials of therapeutics for Duchenne muscular dystrophy (DMD) by characterizing the longitudinal progression of MR biomarkers over 48 months and assessing their relationship to changes in ambulatory clinical function.
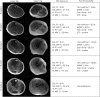
Methods: One hundred sixty participants with DMD were enrolled in this longitudinal, natural history study and underwent MR data acquisition of the lower extremity muscles to determine muscle fat fraction (FF) and MRI T2 biomarkers of disease progression. In addition, 4 tests of ambulatory function were performed. Participants returned for follow-up data collection at 12, 24, 36, and 48 months.
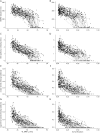
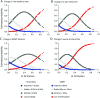
Results: Longitudinal analysis of the MR biomarkers revealed that vastus lateralis FF, vastus lateralis MRI T2, and biceps femoris long head MRI T2 biomarkers were the fastest progressing biomarkers over time in this primarily ambulatory cohort. Biomarker values tended to demonstrate a nonlinear, sigmoidal trajectory over time. The lower extremity biomarkers predicted functional performance 12 and 24 months later, and the magnitude of change in an MR biomarker over time was related to the magnitude of change in function. Vastus lateralis FF, soleus FF, vastus lateralis MRI T2, and biceps femoris long head MRI T2 were the strongest predictors of future loss of function, including loss of ambulation.
Conclusions: This study supports the strong relationship between lower extremity MR biomarkers and measures of clinical function, as well as the ability of MR biomarkers, particularly those from proximal muscles, to predict future ambulatory function and important clinical milestones.
Clinicaltrialsgov identifier: NCT01484678.
© 2020 American Academy of Neurology.
Figures


Comment in
-
The expanding role of MRI in neuromuscular disorders.Nat Rev Neurol. 2020 Jun;16(6):301-302. doi: 10.1038/s41582-020-0346-2. Nat Rev Neurol. 2020. PMID: 32203396 No abstract available.
References
-
- Hoffman EP, Brown RH Jr, Kunkel LM.. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987;51:919–928. - PubMed
-
- Duchenne Muscular Dystrophy and Related Dystrophinopathies: Developing Drugs for Treatment—Guidance for Industry. Silver Spring; US Food and Drug Administration; 2018.
-
- Fleming TR. Surrogate endpoints and FDA's accelerated approval process. Health Aff 2005;24:67–78. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
