Recruitment of Host Nuclear Pore Components to the Vicinity of Theileria Schizonts
- PMID: 32024710
- PMCID: PMC7002307
- DOI: 10.1128/mSphere.00709-19
Recruitment of Host Nuclear Pore Components to the Vicinity of Theileria Schizonts
Abstract
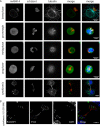
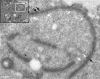
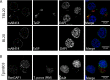
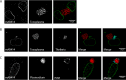
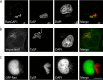
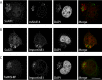
Parasitic protozoans of the genus Theileria are intracellular pathogens that induce the cellular transformation of leukocytes, causing uncontrolled proliferation of the infected host cell. The transforming stage of the parasite has a strictly intracellular lifestyle and ensures its distribution to both daughter cells during host cell cytokinesis by aligning itself across the metaphase plate and by binding tightly to central spindle and astral microtubules. Given the importance of the parasite surface in maintaining interactions with host microtubules, we analyzed the ultrastructure of the host-parasite interface using transmission electron microscopy combined with high-resolution fluorescence microscopy and live-cell imaging. We show that porous membranes, termed annulate lamellae (AL), closely associate with the Theileria surface in infected T cells, B cells, and macrophages and are not detectable in noninfected bovine cell lines such as BL20 or BoMACs. AL are membranous structures found in the cytoplasm of fast-proliferating cells such as cancer cells, oocytes, and embryonic cells. Although AL were first observed more than 60 years ago, the function of these organelles is still not known. Indirect immunofluorescence analysis with a pan-nuclear pore complex antibody, combined with overexpression of a panel of nuclear pore proteins, revealed that the parasite recruits nuclear pore complex components close to its surface. Importantly, we show that, in addition to structural components of the nuclear pore complex, nuclear trafficking machinery, including importin beta 1, RanGAP1, and the small GTPase Ran, also accumulated close to the parasite surface.IMPORTANCETheileria schizonts are the only known eukaryotic organisms capable of transforming another eukaryotic cell; as such, probing of the interactions that occur at the host-parasite interface is likely to lead to novel insights into the cell biology underlying leukocyte proliferation and transformation. Little is known about how the parasite communicates with its host or by what route secreted parasite proteins are translocated into the host, and we propose that nuclear trafficking machinery at the parasite surface might play a role in this. The function of AL remains completely unknown, and our work provides a basis for further investigation into the contribution that these porous, cytomembranous structures might make to the survival of fast-growing transformed cells.
Keywords: Theileria; annulate lamellae; apicomplexan; importin; nuclear pore complex.
Copyright © 2020 Huber et al.
Figures

Similar articles
-
Theileria's Strategies and Effector Mechanisms for Host Cell Transformation: From Invasion to Immortalization.Front Cell Dev Biol. 2021 Apr 20;9:662805. doi: 10.3389/fcell.2021.662805. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33959614 Free PMC article. Review.
-
The Microtubule-Stabilizing Protein CLASP1 Associates with the Theileria annulata Schizont Surface via Its Kinetochore-Binding Domain.mSphere. 2017 Aug 23;2(4):e00215-17. doi: 10.1128/mSphere.00215-17. eCollection 2017 Jul-Aug. mSphere. 2017. PMID: 28861517 Free PMC article.
-
Recruitment of EB1, a master regulator of microtubule dynamics, to the surface of the Theileria annulata schizont.PLoS Pathog. 2013 May;9(5):e1003346. doi: 10.1371/journal.ppat.1003346. Epub 2013 May 9. PLoS Pathog. 2013. PMID: 23675298 Free PMC article.
-
TurboID mapping reveals the exportome of secreted intrinsically disordered proteins in the transforming parasite Theileria annulata.mBio. 2024 Jun 12;15(6):e0341223. doi: 10.1128/mbio.03412-23. Epub 2024 May 15. mBio. 2024. PMID: 38747635 Free PMC article.
-
[What makes a parasite "transforming"? Insights into cancer from the agents of an exotic pathology, Theileria spp].Bull Soc Pathol Exot. 2017 Feb;110(1):55-60. doi: 10.1007/s13149-017-0551-4. Epub 2017 Feb 2. Bull Soc Pathol Exot. 2017. PMID: 28155040 Review. French.
Cited by
-
Theileria parasites sequester host eIF5A to escape elimination by host-mediated autophagy.Nat Commun. 2024 Mar 12;15(1):2235. doi: 10.1038/s41467-024-45022-7. Nat Commun. 2024. PMID: 38472173 Free PMC article.
-
Cytoplasmic nucleoporin assemblage: the cellular artwork in physiology and disease.Nucleus. 2024 Dec;15(1):2387534. doi: 10.1080/19491034.2024.2387534. Epub 2024 Aug 12. Nucleus. 2024. PMID: 39135336 Free PMC article. Review.
-
Modulation of the Host Nuclear Compartment by Trypanosoma cruzi Uncovers Effects on Host Transcription and Splicing Machinery.Front Cell Infect Microbiol. 2021 Oct 19;11:718028. doi: 10.3389/fcimb.2021.718028. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34737973 Free PMC article.
-
Role of Host Small GTPases in Apicomplexan Parasite Infection.Microorganisms. 2022 Jul 7;10(7):1370. doi: 10.3390/microorganisms10071370. Microorganisms. 2022. PMID: 35889089 Free PMC article. Review.
-
Theileria's Strategies and Effector Mechanisms for Host Cell Transformation: From Invasion to Immortalization.Front Cell Dev Biol. 2021 Apr 20;9:662805. doi: 10.3389/fcell.2021.662805. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33959614 Free PMC article. Review.
References
-
- Woods K, von Schubert C, Dobbelaere D. 2013. Hijacking of host cell signaling by Theileria, p 181–197. In Doerig C, Späth G, Wiese M (ed), Protein phosphorylation in parasites: novel targets for antiparasitic intervention. Wiley-VCH Verlag GmbH & Co, Weinheim, Germany.
-
- Dobbelaere D, Baumgartner M. 2009. Theileria, p 613–632. In Schaible UE, Hass A (ed), Intracellular niches of microbes: a pathogens guide through the host cell. Wiley-VCH, Weinheim, Germany.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

