Human T-Cell Lymphotropic Virus Type 1 Transactivator Tax Exploits the XPB Subunit of TFIIH during Viral Transcription
- PMID: 32024775
- PMCID: PMC7108837
- DOI: 10.1128/JVI.02171-19
Human T-Cell Lymphotropic Virus Type 1 Transactivator Tax Exploits the XPB Subunit of TFIIH during Viral Transcription
Abstract
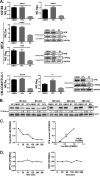
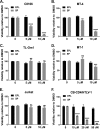
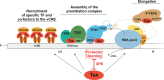
Human T-cell lymphotropic virus type 1 (HTLV-1) Tax oncoprotein is required for viral gene expression. Tax transactivates the viral promoter by recruiting specific transcription factors but also by interfering with general transcription factors involved in the preinitiation step, such as TFIIA and TFIID. However, data are lacking regarding Tax interplay with TFIIH, which intervenes during the last step of preinitiation. We previously reported that XPB, the TFIIH subunit responsible for promoter opening and promoter escape, is required for Tat-induced human-immunodeficiency virus promoter transactivation. Here, we investigated whether XPB may also play a role in HTLV-1 transcription. We report that Tax and XPB directly interact in vitro and that endogenous XPB produced by HTLV-1-infected T cells binds to Tax and is recruited on proviral LTRs. In contrast, XPB recruitment at the LTR is not detected in Tax-negative HTLV-1-infected T cells and is strongly reduced when Tax-induced HTLV-1 LTR transactivation is blocked. XPB overexpression does not affect basal HTLV-1 promoter activation but enhances Tax-mediated transactivation in T cells. Conversely, downregulating XPB strongly reduces Tax-mediated transactivation. Importantly, spironolactone (SP)-mediated inhibition of LTR activation can be rescued by overexpressing XPB but not XPD, another TFIIH subunit. Furthermore, an XPB mutant defective for the ATPase activity responsible for promoter opening does not show rescue of the effect of SP. Finally, XPB downregulation reduces viability of Tax-positive but not Tax-negative HTLV-1-transformed T cell lines. These findings reveal that XPB is a novel cellular cofactor hijacked by Tax to facilitate HTLV-1 transcription.IMPORTANCE HTLV-1 is considered the most potent human oncovirus and is also responsible for severe inflammatory disorders. HTLV-1 transcription is undertaken by RNA polymerase II and is controlled by the viral oncoprotein Tax. Tax transactivates the viral promoter first via the recruitment of CREB and its cofactors to the long terminal repeat (LTR). However, how Tax controls subsequent steps of the transcription process remains unclear. In this study, we explore the link between Tax and the XPB subunit of TFIIH that governs, via its ATPase activity, the promoter-opening step of transcription. We demonstrate that XPB is a novel physical and functional partner of Tax, recruited on HTLV-1 LTR, and required for viral transcription. These findings extend the mechanism of Tax transactivation to the recruitment of TFIIH and reinforce the link between XPB and transactivator-induced viral transcription.
Keywords: TFIIH; oncoprotein; promoter opening; retrovirus; transcription.
Copyright © 2020 American Society for Microbiology.
Figures




Similar articles
-
Novel Interactions between the Human T-Cell Leukemia Virus Type 1 Antisense Protein HBZ and the SWI/SNF Chromatin Remodeling Family: Implications for Viral Life Cycle.J Virol. 2019 Jul 30;93(16):e00412-19. doi: 10.1128/JVI.00412-19. Print 2019 Aug 15. J Virol. 2019. PMID: 31142665 Free PMC article.
-
Hijacking of the O-GlcNAcZYME complex by the HTLV-1 Tax oncoprotein facilitates viral transcription.PLoS Pathog. 2017 Jul 24;13(7):e1006518. doi: 10.1371/journal.ppat.1006518. eCollection 2017 Jul. PLoS Pathog. 2017. PMID: 28742148 Free PMC article.
-
Transactivation of the fucosyltransferase VII gene by human T-cell leukemia virus type 1 Tax through a variant cAMP-responsive element.Blood. 2003 May 1;101(9):3615-21. doi: 10.1182/blood-2002-07-2301. Epub 2002 Dec 27. Blood. 2003. PMID: 12506041
-
[Adult T-cell leukemia induced by HTLV-1: before and after HBZ].Med Sci (Paris). 2010 Apr;26(4):391-6. doi: 10.1051/medsci/2010264391. Med Sci (Paris). 2010. PMID: 20412744 Review. French.
-
Human T cell lymphotropic virus type I genomic expression and impact on intracellular signaling pathways during neurodegenerative disease and leukemia.Front Biosci. 2000 Jan 1;5:D138-68. doi: 10.2741/yao. Front Biosci. 2000. PMID: 10702386 Review.
Cited by
-
Spironolactone and XPB: An Old Drug with a New Molecular Target.Biomolecules. 2020 May 13;10(5):756. doi: 10.3390/biom10050756. Biomolecules. 2020. PMID: 32414008 Free PMC article. Review.
-
Nucleotide Excision Repair: Insights into Canonical and Emerging Functions of the Transcription/DNA Repair Factor TFIIH.Genes (Basel). 2025 Feb 19;16(2):231. doi: 10.3390/genes16020231. Genes (Basel). 2025. PMID: 40004560 Free PMC article. Review.
-
Reduction of antisense transcription affects bovine leukemia virus replication and oncogenesis.PLoS Pathog. 2024 Nov 7;20(11):e1012659. doi: 10.1371/journal.ppat.1012659. eCollection 2024 Nov. PLoS Pathog. 2024. PMID: 39509441 Free PMC article.
-
Transactivation of the novel 5' cis-acting element of mouse mammary tumor virus (MMTV) by human retroviral transactivators Tat and Tax.Commun Biol. 2024 Nov 16;7(1):1521. doi: 10.1038/s42003-024-07139-9. Commun Biol. 2024. PMID: 39550519 Free PMC article.
-
Current State of Therapeutics for HTLV-1.Viruses. 2024 Oct 15;16(10):1616. doi: 10.3390/v16101616. Viruses. 2024. PMID: 39459949 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

