Osteoclast-mediated bone resorption is controlled by a compensatory network of secreted and membrane-tethered metalloproteinases
- PMID: 32024800
- PMCID: PMC7603424
- DOI: 10.1126/scitranslmed.aaw6143
Osteoclast-mediated bone resorption is controlled by a compensatory network of secreted and membrane-tethered metalloproteinases
Abstract
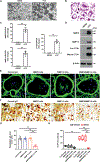
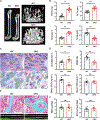
Osteoclasts actively remodel both the mineral and proteinaceous components of bone during normal growth and development as well as pathologic states ranging from osteoporosis to bone metastasis. The cysteine proteinase cathepsin K confers osteoclasts with potent type I collagenolytic activity; however, cathepsin K-null mice, as well as cathepsin K-mutant humans, continue to remodel bone and degrade collagen by as-yet-undefined effectors. Here, we identify a cathepsin K-independent collagenolytic system in osteoclasts that is composed of a functionally redundant network of the secreted matrix metalloproteinase MMP9 and the membrane-anchored matrix metalloproteinase MMP14. Unexpectedly, whereas deleting either of the proteinases individually leaves bone resorption intact, dual targeting of Mmp9 and Mmp14 inhibited the resorptive activity of mouse osteoclasts in vitro and in vivo and human osteoclasts in vitro. In vivo, Mmp9/Mmp14 conditional double-knockout mice exhibited marked increases in bone density and displayed a highly protected status against either parathyroid hormone- or ovariectomy-induced pathologic bone loss. Together, these studies characterize a collagenolytic system operative in mouse and human osteoclasts and identify the MMP9/MMP14 axis as a potential target for therapeutic interventions for bone-wasting disease states.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures






References
-
- Zaidi M, Skeletal remodeling in health and disease. Nat. Med 13, 791–801 (2007). - PubMed
-
- Teitelbaum SL, Ross FP, Genetic regulation of osteoclast development and function. Nat. Rev. Genet 4, 638–649 (2003). - PubMed
-
- Boyle WJ, Simonet WS, Lacey DL, Osteoclast differentiation and activation. Nature 423, 337–342 (2003). - PubMed
-
- Takayanagi H, Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol 7, 292–304 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

