GloBody Technology: Detecting Anti-Drug Antibody against VH/VL domains
- PMID: 32024871
- PMCID: PMC7002611
- DOI: 10.1038/s41598-020-58041-3
GloBody Technology: Detecting Anti-Drug Antibody against VH/VL domains
Abstract
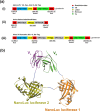
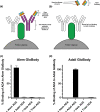
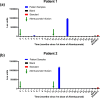
The occurrence of anti-drug antibodies following administration of therapeutic monoclonal antibody to patients is a growing problem that is attracting attention from frontline clinicians. Ideally, an initial indicative point of care test would provide guidance to seek testing approved by the regulatory authorities. Here we describe a platform for the detection of IgG anti-drug antibodies that may provide an initial screen for all therapeutic monoclonal antibodies. Synthetic genes encoding Nanoluciferase polypeptides were inserted between the variable heavy and light domain encoding region of known antibody drugs (alemtuzumab and adalimumab) to generate recombinant single chain GloBodies, which retain the drug antibody paratopes and Nanoluciferase activity. In the presence of anti-drug antibodies, the GloBody is bound by specific IgG in the sample. These complexes are captured on immobilised Protein G and the luciferase activity determined. The amount of light generated being indicative of the anti-drug IgG antibody levels in serum. It should be possible to assemble GloBody reagents for all therapeutic monoclonal antibodies and adapt the capture phase to include additional specific isotypes. The assay has the potential to be developed for use with a drop of blood allowing initial pre-screening in a point of care setting.
Conflict of interest statement
AK has trademarked GloBody and filed patents for potential commercial development related to the GloBody ADA technology. GG, SG, KS have received fees for consultancy, meetings and grant support (SG) from Sanofi Genzyme within the last three years, otherwise none are considered relevant. However, SG has received travel support, consultancy fees or grant support from Biogen, Novartis, Teva, Pfizer, and Takeda. DB has received consultancy and presentation fees from Canbex Therapeutics, Japan Tobacco, Merck and Roche. KS has consultancy and presentation fees from Biogen, Bayer HealthCare, Lipomed, Medday, Merck, Novartis, Roche and Teva. GG has received consultancy, presentation fees or grants from AbbVie Biotherapeutics, Bayer Healthcare, Biogen, Canbex, Celgene, Ironwood, Japan Tobacco, Merck, Novartis, Roche, Sanofi-Genzyme, Synthon, Takeda, Teva and Vertex.
Figures
Similar articles
-
Evaluation of an immunoassay for human-specific quantitation of therapeutic antibodies in serum samples from non-human primates.J Pharm Biomed Anal. 2009 May 1;49(4):1003-8. doi: 10.1016/j.jpba.2009.01.030. Epub 2009 Feb 5. J Pharm Biomed Anal. 2009. PMID: 19250787
-
Characterization of murine anti-human Fab antibodies for use in an immunoassay for generic quantification of human Fab fragments in non-human serum samples including cynomolgus monkey samples.J Pharm Biomed Anal. 2013 Jan;72:208-15. doi: 10.1016/j.jpba.2012.08.023. Epub 2012 Sep 7. J Pharm Biomed Anal. 2013. PMID: 23017233
-
A systematic study of the effect of low pH acid treatment on anti-drug antibodies specific for a domain antibody therapeutic: Impact on drug tolerance, assay sensitivity and post-validation method assessment of ADA in clinical serum samples.J Immunol Methods. 2017 Sep;448:91-104. doi: 10.1016/j.jim.2017.06.002. Epub 2017 Jun 16. J Immunol Methods. 2017. PMID: 28625864
-
Engineered antibody fragments and the rise of single domains.Nat Biotechnol. 2005 Sep;23(9):1126-36. doi: 10.1038/nbt1142. Nat Biotechnol. 2005. PMID: 16151406 Review.
-
Fabrication of Fragment Antibody-Enzyme Complex as a Sensing Element for Immunosensing.Int J Mol Sci. 2022 Jan 25;23(3):1335. doi: 10.3390/ijms23031335. Int J Mol Sci. 2022. PMID: 35163258 Free PMC article. Review.
Cited by
-
COVID-19 Vaccine Response in People with Multiple Sclerosis.Ann Neurol. 2022 Jan;91(1):89-100. doi: 10.1002/ana.26251. Epub 2021 Nov 17. Ann Neurol. 2022. PMID: 34687063 Free PMC article.
-
The Irony of Humanization: Alemtuzumab, the First, But One of the Most Immunogenic, Humanized Monoclonal Antibodies.Front Immunol. 2020 Feb 14;11:124. doi: 10.3389/fimmu.2020.00124. eCollection 2020. Front Immunol. 2020. PMID: 32117274 Free PMC article.
-
A cell-based assay for the detection of neutralizing antibodies against alemtuzumab.Biotechniques. 2020 Apr;68(4):185-190. doi: 10.2144/btn-2019-0122. Epub 2020 Feb 25. Biotechniques. 2020. PMID: 32096651 Free PMC article.
-
Detecting and predicting neutralization of alemtuzumab responses in MS.Neurol Neuroimmunol Neuroinflamm. 2020 Jun 4;7(4):e767. doi: 10.1212/NXI.0000000000000767. Print 2020 Jul. Neurol Neuroimmunol Neuroinflamm. 2020. PMID: 32499328 Free PMC article.
References
-
- Smith HW, Butterfield A, Sun D. Detection ofantibodies against therapeutic proteins in the presence of residual protein using solidphase extraction with acid dissociation (SPEAD) sample treatment prior to ELISA. Regul. Toxicol. Pharmacol. 2007;49:230–237. doi: 10.1016/j.yrtph.2007.07.005. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

