The deubiquitinase OTUB1 augments NF-κB-dependent immune responses in dendritic cells in infection and inflammation by stabilizing UBC13
- PMID: 32024978
- PMCID: PMC8167118
- DOI: 10.1038/s41423-020-0362-6
The deubiquitinase OTUB1 augments NF-κB-dependent immune responses in dendritic cells in infection and inflammation by stabilizing UBC13
Abstract
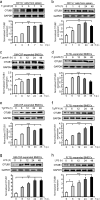
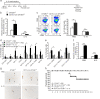
Dendritic cells (DCs) are indispensable for defense against pathogens but may also contribute to immunopathology. Activation of DCs upon the sensing of pathogens by Toll-like receptors (TLRs) is largely mediated by pattern recognition receptor/nuclear factor-κB (NF-κB) signaling and depends on the appropriate ubiquitination of the respective signaling molecules. However, the ubiquitinating and deubiquitinating enzymes involved and their interactions are only incompletely understood. Here, we reveal that the deubiquitinase OTU domain, ubiquitin aldehyde binding 1 (OTUB1) is upregulated in DCs upon murine Toxoplasma gondii infection and lipopolysaccharide challenge. Stimulation of DCs with the TLR11/12 ligand T. gondii profilin and the TLR4 ligand lipopolysaccharide induced an increase in NF-κB activation in OTUB1-competent cells, resulting in elevated interleukin-6 (IL-6), IL-12, and tumor necrosis factor (TNF) production, which was also observed upon the specific stimulation of TLR2, TLR3, TLR7, and TLR9. Mechanistically, OTUB1 promoted NF-κB activity in DCs by K48-linked deubiquitination and stabilization of the E2-conjugating enzyme UBC13, resulting in increased K63-linked ubiquitination of IRAK1 (IL-1 receptor-associated kinase 1) and TRAF6 (TNF receptor-associated factor 6). Consequently, DC-specific deletion of OTUB1 impaired the production of cytokines, in particular IL-12, by DCs over the first 2 days of T. gondii infection, resulting in the diminished production of protective interferon-γ (IFN-γ) by natural killer cells, impaired control of parasite replication, and, finally, death from chronic T. encephalitis, all of which could be prevented by low-dose IL-12 treatment in the first 3 days of infection. In contrast, impaired OTUB1-deficient DC activation and cytokine production by OTUB1-deficient DCs protected mice from lipopolysaccharide-induced immunopathology. Collectively, these findings identify OTUB1 as a potent novel regulator of DCs during infectious and inflammatory diseases.
Keywords: OTUB1; dendritic cell; innate immunity; signal transduction; ubiquitination.
Conflict of interest statement
The authors declare no competing interests.
Figures




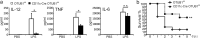
References
-
- Yarovinsky F, et al. TLR11 activation of dendritic cells by a protozoan Profilin-like protein. Science (80-.). 2005;308:1626–1629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

