The guidance receptor plexin D1 is a mechanosensor in endothelial cells
- PMID: 32025034
- PMCID: PMC7025890
- DOI: 10.1038/s41586-020-1979-4
The guidance receptor plexin D1 is a mechanosensor in endothelial cells
Erratum in
-
Author Correction: The guidance receptor plexin D1 is a mechanosensor in endothelial cells.Nature. 2022 May;605(7910):E6. doi: 10.1038/s41586-022-04815-w. Nature. 2022. PMID: 35504972 No abstract available.
Abstract
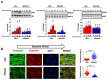
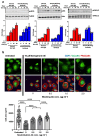
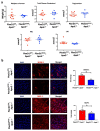
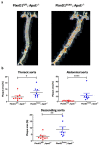
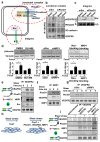
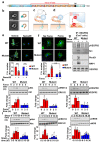
Shear stress on arteries produced by blood flow is important for vascular development and homeostasis but can also initiate atherosclerosis1. Endothelial cells that line the vasculature use molecular mechanosensors to directly detect shear stress profiles that will ultimately lead to atheroprotective or atherogenic responses2. Plexins are key cell-surface receptors of the semaphorin family of cell-guidance signalling proteins and can regulate cellular patterning by modulating the cytoskeleton and focal adhesion structures3-5. However, a role for plexin proteins in mechanotransduction has not been examined. Here we show that plexin D1 (PLXND1) has a role in mechanosensation and mechanically induced disease pathogenesis. PLXND1 is required for the response of endothelial cells to shear stress in vitro and in vivo and regulates the site-specific distribution of atherosclerotic lesions. In endothelial cells, PLXND1 is a direct force sensor and forms a mechanocomplex with neuropilin-1 and VEGFR2 that is necessary and sufficient for conferring mechanosensitivity upstream of the junctional complex and integrins. PLXND1 achieves its binary functions as either a ligand or a force receptor by adopting two distinct molecular conformations. Our results establish a previously undescribed mechanosensor in endothelial cells that regulates cardiovascular pathophysiology, and provide a mechanism by which a single receptor can exhibit a binary biochemical nature.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

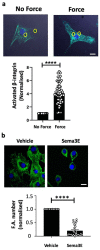
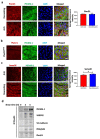
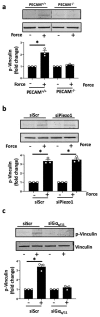
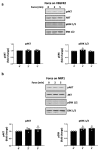
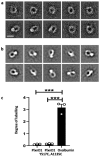
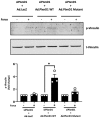
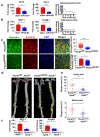
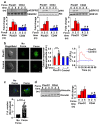
Comment in
-
Plexin D1 is a mechanosensor that regulates the site-specific distribution of atherosclerosis.Nat Rev Cardiol. 2020 Apr;17(4):199. doi: 10.1038/s41569-020-0355-2. Nat Rev Cardiol. 2020. PMID: 32099089 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

