"Dysfunctions" induced by Roux-en-Y gastric bypass surgery are concomitant with metabolic improvement independent of weight loss
- PMID: 32025334
- PMCID: PMC6985254
- DOI: 10.1038/s41421-019-0138-2
"Dysfunctions" induced by Roux-en-Y gastric bypass surgery are concomitant with metabolic improvement independent of weight loss
Erratum in
-
Erratum: Author Correction: "Dysfunctions" induced by Roux-en-Y gastric bypass surgery are concomitant with metabolic improvement independent of weight loss.Cell Discov. 2020 Apr 28;6:25. doi: 10.1038/s41421-020-0163-1. eCollection 2020. Cell Discov. 2020. PMID: 32351705 Free PMC article.
Abstract
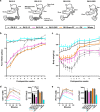
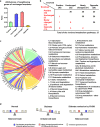
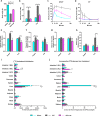
Metabolic surgery has been increasingly recommended for obese diabetic patients, but questions remain as to its molecular mechanism that leads to improved metabolic parameters independently of weight loss from a network viewpoint. We evaluated the role of the Roux limb (RL) in Roux-en-Y gastric bypass (RYGB) surgery in nonobese diabetic rat models. Improvements in metabolic parameters were greater in the long-RL RYGB group. Transcriptome profiles reveal that amelioration of diabetes state following RYGB differs remarkably from both normal and diabetic states. According to functional analysis, RYGB surgery significantly affected a major gene group, i.e., the newly changed group, which represented diabetes-irrelevant genes abnormally expressed after RYGB. We hypothesize that novel "dysfunctions" carried by this newly changed gene group induced by RYGB rebalance diabetic states and contribute to amelioration of metabolic parameters. An unusual increase in cholesterol (CHOL) biosynthesis in RL enriched by the newly changed group was concomitant with ameliorated metabolic parameters, as demonstrated by measurements of physiological parameters and biodistribution analysis using [14C]-labeled glucose. Our findings demonstrate RYGB-induced "dysfunctions" in the newly changed group as a compensatory role contributes to amelioration of diabetes. Rather than attempting to normalize "abnormal" molecules, we suggest a new disease treatment strategy of turning "normal" molecules "abnormal" in order to achieve a new "normal" physiological balance. It further implies a novel strategy for drug discovery, i.e. targeting also on "normal" molecules, which are traditionally ignored in pharmaceutical development.
Keywords: Mechanisms of disease; Transcriptomics.
© The Author(s) 2020.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

