Machine-driven parameter screen of biochemical reactions
- PMID: 32025730
- PMCID: PMC7144897
- DOI: 10.1093/nar/gkaa079
Machine-driven parameter screen of biochemical reactions
Abstract
The development of complex methods in molecular biology is a laborious, costly, iterative and often intuition-bound process where optima are sought in a multidimensional parameter space through step-by-step optimizations. The difficulty of miniaturizing reactions under the microliter volumes usually handled in multiwell plates by robots, plus the cost of the experiments, limit the number of parameters and the dynamic ranges that can be explored. Nevertheless, because of non-linearities of the response of biochemical systems to their reagent concentrations, broad dynamic ranges are necessary. Here we use a high-performance nanoliter handling platform and computer generation of liquid transfer programs to explore in quadruplicates 648 combinations of 4 parameters of a biochemical reaction, the reverse-transcription, which lead us to uncover non-linear responses, parameter interactions and novel mechanistic insights. With the increased availability of computer-driven laboratory platforms for biotechnology, our results demonstrate the feasibility and advantage of methods development based on reproducible, computer-aided exhaustive characterization of biochemical systems.
© The Author(s) 2020. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




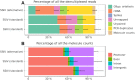
References
-
- Genot A.J., Baccouche A., Sieskind R., Aubert-Kato N., Bredeche N., Bartolo J.F., Taly V., Fujii T., Rondelez Y.. High-resolution mapping of bifurcations in nonlinear biochemical circuits. Nat. Chem. 2016; 8:760–767. - PubMed
-
- Zucha D., Androvic P., Kubista M., Valihrach L.. Performance comparison of reverse transcriptases for single-cell studies. Clinical Chemistry. 2020; 66:217–228. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

