Genetics of the congenital absence of the vas deferens
- PMID: 32025909
- PMCID: PMC7864840
- DOI: 10.1007/s00439-020-02122-w
Genetics of the congenital absence of the vas deferens
Abstract
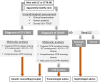
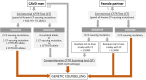
Congenital absence of the vas deferens (CAVD) may have various clinical presentations depending on whether it is bilateral (CBAVD) or unilateral (CUAVD), complete or partial, and associated or not with other abnormalities of the male urogenital tract. CBAVD is usually discovered in adult men either during the systematic assessment of cystic fibrosis or other CFTR-related conditions, or during the exploration of isolated infertility with obstructive azoospermia. The prevalence of CAVDs in men is reported to be approximately 0.1%. However, this figure is probably underestimated, because unilateral forms of CAVD in asymptomatic fertile men are not usually diagnosed. The diagnosis of CAVDs is based on clinical, ultrasound, and sperm examinations. The majority of subjects with CAVD carry at least one cystic fibrosis-causing mutation that warrants CFTR testing and in case of a positive result, genetic counseling prior to conception. Approximately 2% of the cases of CAVD are hemizygous for a loss-of-function mutation in the ADGRG2 gene that may cause a familial form of X-linked infertility. However, despite this recent finding, 10-20% of CBAVDs and 60-70% of CUAVDs remain without a genetic diagnosis. An important proportion of these unexplained CAVDs coexist with a solitary kidney suggesting an early organogenesis disorder (Wolffian duct), unlike CAVDs related to CFTR or ADGRG2 mutations, which might be the result of progressive degeneration that begins later in fetal life and probably continues after birth. How the dysfunction of CFTR, ADGRG2, or other genes such as SLC29A3 leads to this involution is the subject of various pathophysiological hypotheses that are discussed in this review.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

