Safety of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis: Experience from 92 Sites in an Open-Label US Expanded Access Program
- PMID: 32026347
- PMCID: PMC6964201
- DOI: 10.1007/s41030-017-0049-z
Safety of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis: Experience from 92 Sites in an Open-Label US Expanded Access Program
Abstract
Introduction: In phase 3 clinical trials, pirfenidone significantly slowed disease progression with a well-defined and medically manageable safety profile in patients with idiopathic pulmonary fibrosis (IPF). This study examined safety events related to pirfenidone in patients with IPF in an expanded access program in the US.
Methods: The Expanded Access Program allowed patients with IPF access to pirfenidone prior to US Food and Drug Administration approval. Patients had an IPF diagnosis including a definite or possible usual interstitial pneumonia (UIP) pattern, predicted forced vital capacity ≥50%, and predicted diffusing capacity for carbon monoxide ≥30%. Clinical laboratory data and adverse drug reactions (ADRs) deemed causally related to pirfenidone were analyzed using descriptive summary statistics.
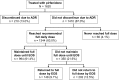
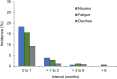
Results: Of the 1620 patients treated, 1221 (75.4%) completed the program: 66.5% had definite UIP, and 33.2% had possible UIP. Mean (SD) pirfenidone exposure was 22.8 (9.6) weeks, and mean (SD) daily dose during the course of treatment was 2058.7 (399.2) mg. ADRs occurred in 64.9% of patients: 3.3% were severe and 0.2% life threatening. The most common ADRs were nausea (22.6%) and fatigue (19.6%); 13.0% of patients discontinued due to ADRs. Serious ADRs occurred in 24 patients (1.5%), which were primarily related to elevated liver function enzymes (ten patients, 0.6%). No ADRs led to death.
Conclusions: In this open-label study of 1620 patients with IPF, including those with possible UIP, the safety profile of pirfenidone was consistent with that of earlier clinical trials, and no new safety signals were identified. NCT02141087.
Funding: Genentech, Inc., a member of the Roche group.
Keywords: Idiopathic pulmonary fibrosis; Pirfenidone; Safety.
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Medical