A Randomized, Double-Blind, Placebo-Controlled Study to Assess the Efficacy and Safety of Ambroxol Hard-Boiled Lozenges in Patients with Acute Pharyngitis
- PMID: 32026411
- PMCID: PMC6966982
- DOI: 10.1007/s41030-019-00100-w
A Randomized, Double-Blind, Placebo-Controlled Study to Assess the Efficacy and Safety of Ambroxol Hard-Boiled Lozenges in Patients with Acute Pharyngitis
Abstract
Introduction: The aim of this study was to evaluate the efficacy and safety of a new hard-boiled lozenge formulation containing ambroxol 20 mg versus placebo for the relief of sore throat in patients with acute pharyngitis.
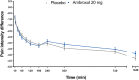
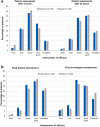
Methods: This was a phase 3, randomized, double-blind, placebo-controlled, parallel-group multicenter trial conducted between June and September 2018 in South Africa. Patients with a diagnosis of acute pharyngitis, onset ≤ 72 h, and sore throat pain of at least moderate intensity were randomized to receive either ambroxol 20 mg or placebo hard-boiled lozenges. The primary efficacy endpoint was the normalized time-weighted sum of pain intensity differences (SPID) from baseline over 3 h following administration of the first lozenge (SPIDnorm,0-3h). Secondary efficacy endpoints included SPID 24 h after the first lozenge intake (SPIDnorm,0-24h) and patient assessment of efficacy at 3 and 24 h after the first lozenge.
Results: Of 422 patients from 11 centers, 390 were randomized to one of the two treatment groups (n = 196, ambroxol; n = 194, placebo) and 388 were analyzed (modified intention-to-treat). The mean ± standard deviation SPIDnorm,0-3h values were -0.386 (0.259) and -0.366 (0.243) in the ambroxol and placebo groups, respectively, and the adjusted mean ± standard error SPIDnorm0-3h difference between ambroxol and placebo was -0.020 (0.025) (p = 0.443). Comparable results between treatment groups were also found for SPIDnorm,0-24h and patient assessment of efficacy at 3 and 24 h after the first lozenge. The incidence of treatment-emergent adverse events (TEAEs) was similar between treatment groups (11.7% for ambroxol versus 9.3% for placebo).
Conclusion: Although marked pain relief was observed over the first 3 h of treatment, superiority of ambroxol 20 mg hard-boiled lozenges versus placebo was not demonstrated in this study.
Trial registration: NCT03583658.
Funding: Sanofi-Aventis Group.
Keywords: Acute pharyngitis; Ambroxol; Local anesthetic; Lozenges; Sore throat.
Figures



References
-
- Palm J, Fuchs K, Stammer H, Schumacher-Stimpfl A, Milde J. Efficacy and safety of a triple active sore throat lozenge in the treatment of patients with acute pharyngitis: results of a multi-centre, randomised, placebo-controlled, double-blind, parallel-group trial (DoriPha) Int J Clin Pract. 2018;72(12):17. doi: 10.1111/ijcp.13272. - DOI - PMC - PubMed
-
- Fischer J, Pschorn U, Vix JM, Peil H, Aicher B, Muller A, de Mey C. Efficacy and tolerability of ambroxol hydrochloride lozenges in sore throat. Randomised, double-blind, placebo-controlled trials regarding the local anaesthetic properties. Arzneimittelforschung. 2002;52(4):256–263. - PubMed
-
- McNally D, Shephard A, Field E. Randomised, double-blind, placebo-controlled study of a single dose of an amylmetacresol/2,4-dichlorobenzyl alcohol plus lidocaine lozenge or a hexylresorcinol lozenge for the treatment of acute sore throat due to upper respiratory tract infection. J Pharm Pharm Sci. 2012;15(2):281–294. doi: 10.18433/J31309. - DOI - PubMed
-
- Bouroubi A, Donazzolo Y, Donath F, Eccles R, Russo M, Harambillet N, Gautier S, Montagne A. Pain relief of sore throat with a new anti-inflammatory throat lozenge, ibuprofen 25 mg: a randomised, double-blind, placebo-controlled, international phase III study. Int J Clin Pract. 2017;71(9):4. doi: 10.1111/ijcp.12961. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

