Neurocognitive and psychiatric disorders-related axonal degeneration in Parkinson's disease
- PMID: 32026517
- PMCID: PMC7154645
- DOI: 10.1002/jnr.24584
Neurocognitive and psychiatric disorders-related axonal degeneration in Parkinson's disease
Abstract
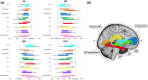
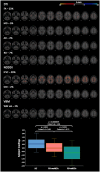
Neurocognitive and psychiatric disorders have significant consequences for quality of life in patients with Parkinson's disease (PD). In the current study, we evaluated microstructural white matter (WM) alterations associated with neurocognitive and psychiatric disorders in PD using neurite orientation dispersion and density imaging (NODDI) and linked independent component analysis (LICA). The indices of NODDI were compared between 20 and 19 patients with PD with and without neurocognitive and psychiatric disorders, respectively, and 25 healthy controls using tract-based spatial statistics and tract-of-interest analyses. LICA was applied to model inter-subject variability across measures. A widespread reduction in axonal density (indexed by intracellular volume fraction [ICVF]) was demonstrated in PD patients with and without neurocognitive and psychiatric disorders, as compared with healthy controls. Compared with patients without neurocognitive and psychiatric disorders, patients with neurocognitive and psychiatric disorders exhibited more extensive (posterior predominant) decreases in axonal density. Using LICA, ICVF demonstrated the highest contribution (59% weight) to the main effects of diagnosis that reflected widespread decreases in axonal density. These findings suggest that axonal loss is a major factor underlying WM pathology related to neurocognitive and psychiatric disorders in PD, whereas patients with neurocognitive and psychiatric disorders had broader axonal pathology, as compared with those without. LICA suggested that the ICVF can be used as a useful biomarker of microstructural changes in the WM related to neurocognitive and psychiatric disorders in PD.
Keywords: Parkinson's disease; axons; biomarkers; diffusion tensor imaging; linked independent component analysis; neurite orientation dispersion and density imaging.
© 2020 The Authors. Journal of Neuroscience Research published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

Similar articles
-
Local white matter abnormalities in Parkinson's disease with mild cognitive impairment: Assessed with neurite orientation dispersion and density imaging.J Neurosci Res. 2023 Jul;101(7):1154-1169. doi: 10.1002/jnr.25179. Epub 2023 Feb 28. J Neurosci Res. 2023. PMID: 36854050
-
Multifocal alterations of white matter accompany the transition from normal cognition to dementia in Parkinson's disease patients.Brain Imaging Behav. 2019 Feb;13(1):232-240. doi: 10.1007/s11682-018-9863-7. Brain Imaging Behav. 2019. PMID: 29629498
-
Application of neurite orientation dispersion and density imaging to characterize brain microstructural abnormalities in type-2 diabetics with mild cognitive impairment.J Magn Reson Imaging. 2019 Sep;50(3):889-898. doi: 10.1002/jmri.26687. Epub 2019 Feb 19. J Magn Reson Imaging. 2019. PMID: 30779402
-
Influence of diabetes mellitus on longitudinal atrophy and cognition in Parkinson's disease.J Neurol Sci. 2017 Jun 15;377:122-126. doi: 10.1016/j.jns.2017.04.010. Epub 2017 Apr 11. J Neurol Sci. 2017. PMID: 28477681
-
Neurite Orientation Dispersion and Density Imaging in Psychiatric Disorders: A Systematic Literature Review and a Technical Note.Biol Psychiatry Glob Open Sci. 2022 Jan 21;3(1):10-21. doi: 10.1016/j.bpsgos.2021.12.012. eCollection 2023 Jan. Biol Psychiatry Glob Open Sci. 2022. PMID: 36712566 Free PMC article. Review.
Cited by
-
White matter changes in Parkinson's disease.NPJ Parkinsons Dis. 2023 Oct 31;9(1):150. doi: 10.1038/s41531-023-00592-z. NPJ Parkinsons Dis. 2023. PMID: 37907554 Free PMC article. Review.
-
Brain white matter changes and their associations with non-motor dysfunction in orthostatic hypotension in α-synucleinopathy: A NODDI study.CNS Neurosci Ther. 2024 Apr;30(4):e14712. doi: 10.1111/cns.14712. CNS Neurosci Ther. 2024. PMID: 38615364 Free PMC article.
-
The role of brain perivascular space burden in early-stage Parkinson's disease.NPJ Parkinsons Dis. 2021 Feb 5;7(1):12. doi: 10.1038/s41531-021-00155-0. NPJ Parkinsons Dis. 2021. PMID: 33547311 Free PMC article.
-
Cingulum and Uncinate Fasciculus Microstructural Abnormalities in Parkinson's Disease: A Systematic Review of Diffusion Tensor Imaging Studies.Biology (Basel). 2023 Mar 20;12(3):475. doi: 10.3390/biology12030475. Biology (Basel). 2023. PMID: 36979166 Free PMC article. Review.
-
Cortical microstructural alterations in different stages of Parkinson's disease.Brain Imaging Behav. 2024 Dec;18(6):1438-1447. doi: 10.1007/s11682-024-00931-5. Epub 2024 Sep 27. Brain Imaging Behav. 2024. PMID: 39331345
References
-
- Andica, C. , Kamagata, K. , Hatano, T. , Okuzumi, A. , Saito, A. , Nakazawa, M. , … Aoki, S. (2018). Neurite orientation dispersion and density imaging of the nigrostriatal pathway in Parkinson's disease: Retrograde degeneration observed by tract‐profile analysis. Parkinsonism & Related Disorders, 51, 55–60. 10.1016/j.parkreldis.2018.02.046 - DOI - PubMed
-
- Aquino, D. , Contarino, V. , Albanese, A. , Minati, L. , Farina, L. , Grisoli, M. , … Chiapparini, L. (2014). Substantia nigra in Parkinson's disease: A multimodal MRI comparison between early and advanced stages of the disease. Neurological Sciences, 35(5), 753–758. 10.1007/s10072-013-1595-2 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

