Multi-Stimuli Responsive FRET Processes of Bifluorophoric AIEgens in an Amphiphilic Copolymer and Its Application to Cyanide Detection in Aqueous Media
- PMID: 32026696
- PMCID: PMC7325583
- DOI: 10.1021/acsami.9b21970
Multi-Stimuli Responsive FRET Processes of Bifluorophoric AIEgens in an Amphiphilic Copolymer and Its Application to Cyanide Detection in Aqueous Media
Abstract
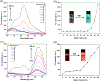
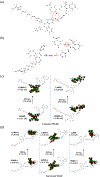
A novel amphiphilic aggregation-induced emission (AIE) copolymer, that is, poly(NIPAM-co-TPE-SP), consisting of N-isopropylacrylamide (NIPAM) as a hydrophilic unit and a tetraphenylethylene-spiropyran monomer (TPE-SP) as a bifluorophoric unit is reported. Upon UV exposure, the close form of non-emissive spiropyran (SP) in poly(NIPAM-co-TPE-SP) can be photo-switched to the open form of emissive merocyanine (MC) in poly(NIPAM-co-TPE-MC) in an aqueous solution, leading to ratiometric fluorescence of AIEgens between green TPE and red MC emissions at 517 and 627 nm, respectively, via Förster resonance energy transfer (FRET). Distinct FRET processes of poly(NIPAM-co-TPE-MC) can be observed under various UV and visible light irradiations, acid-base conditions, thermal treatments, and cyanide ion interactions, which are also confirmed by theoretical studies. The subtle perturbations of environmental factors, such as UV exposure, pH value, temperature, and cyanide ion, can be detected in aqueous media by distinct ratiometric fluorescence changes of the FRET behavior in the amphiphilic poly(NIPAM-co-TPE-MC). Moreover, the first FRET sensor polymer poly(NIPAM-co-TPE-MC) based on dual AIEgens of TPE and MC units is developed to show a very high selectivity and sensitivity with a low detection limit (LOD = 0.26 μM) toward the cyanide ion in water, which only contain an approximately 1% molar ratio of the bifluorophoric content and can be utilized in cellular bioimaging applications for cyanide detections.
Keywords: Förster resonance energy transfer (FRET); aggregation-induced emission (AIE); cyanide; spiropyran; tetraphenylethylene.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

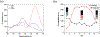
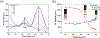



References
-
- Kulig KW; Ballantyne B Cyanide Toxicity; US Department of Health & Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, 1991.
-
- Wang F; Wang L; Chen X; Yoon J Recent Progress in the Development of Fluorometric and Colorimetric Chemosensors for Detection of Cyanide Ions. Chem. Soc. Rev. 2014, 43, 4312–4324. - PubMed
-
- Gale PA; Caltagirone C Anion Sensing by Small Molecules and Molecular Ensembles. Chem. Soc. Rev. 2015, 44, 4212–4227. - PubMed
-
- World Health Organization Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2008.
-
- Yang L; Li X; Qu Y; Qu W; Zhang X; Hang Y; Ågren H; Hua J Red Turn-on Fluorescent Phenazine-Greenine Chemodosimeters for Cyanide Anion in Aqueous Solution and Its Application for Cell Imaging. Sens. Actuators, B 2014, 203, 833–847.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

