Old but not obsolete: an enhanced high-speed immunoblot
- PMID: 32027361
- PMCID: PMC7324151
- DOI: 10.1093/jb/mvaa016
Old but not obsolete: an enhanced high-speed immunoblot
Erratum in
-
Old but not obsolete: an enhanced high-speed immunoblot.J Biochem. 2020 Sep 1;168(3):313. doi: 10.1093/jb/mvaa078. J Biochem. 2020. PMID: 32905599 Free PMC article. No abstract available.
Abstract
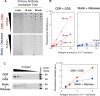
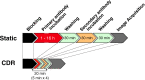
The immunoblotting technique (also known as western blotting) is an essential tool used in biomedical research to determine the relative size and abundance of specific proteins and protein modifications. However, long incubation times severely limit its throughput. We have devised a system that improves antigen binding by cyclic draining and replenishing (CDR) of the antibody solution in conjunction with an immunoreaction enhancing agent. Biochemical analyses revealed that the CDR method reduced the incubation time of the antibodies, and the presence of a commercial immunoreaction enhancing agent altered the affinity of the antibody, respectively. Combination of the CDR method with the immunoreaction enhancing agent considerably enhanced the output signal and further reduced the incubation time of the antibodies. The resulting high-speed immunoblot can be completed in 20 min without any loss in sensitivity. Further, the antibodies are fully reusable. This method is effective for both chemiluminescence and fluorescence detection. Widespread adoption of this technique could dramatically boost efficiency and productivity across the life sciences.
Keywords: chemiluminescence; fluorescence; immunoblot; immunoreaction enhancing agent; western blot.
Published by Oxford University Press on behalf of the Japanese Biochemical Society 2020. This work is written by US Government employees and is in the public domain in the US.
Figures





References
-
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227, 680–685 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

