Anti-vascular endothelial growth factor for neovascular glaucoma
- PMID: 32027392
- PMCID: PMC7003996
- DOI: 10.1002/14651858.CD007920.pub3
Anti-vascular endothelial growth factor for neovascular glaucoma
Update in
-
Anti-vascular endothelial growth factor for neovascular glaucoma.Cochrane Database Syst Rev. 2023 Apr 3;4(4):CD007920. doi: 10.1002/14651858.CD007920.pub4. Cochrane Database Syst Rev. 2023. PMID: 37010901 Free PMC article.
Abstract
Background: Neovascular glaucoma (NVG) is a potentially blinding, secondary glaucoma. It is caused by the formation of abnormal new blood vessels, which prevent normal drainage of aqueous from the anterior segment of the eye. Anti-vascular endothelial growth factor (anti-VEGF) medications are specific inhibitors of the primary mediators of neovascularization. Studies have reported the effectiveness of anti-VEGF medications for the control of intraocular pressure (IOP) in NVG.
Objectives: To assess the effectiveness of intraocular anti-VEGF medications, alone or with one or more type of conventional therapy, compared with no anti-VEGF medications for the treatment of NVG.
Search methods: We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register); MEDLINE; Embase; PubMed; and LILACS to 22 March 2019; metaRegister of Controlled Trials to 13 August 2013; and two additional trial registers to 22 March 2019. We did not use any date or language restrictions in the electronic search for trials.
Selection criteria: We included randomised controlled trials (RCTs) of people treated with anti-VEGF medications for NVG.
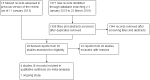
Data collection and analysis: Two review authors independently assessed the search results for trials, extracted data, and assessed risk of bias, and the certainty of the evidence. We resolved discrepancies through discussion.
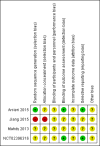
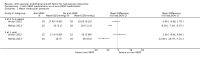
Main results: We included four RCTs (263 participants) and identified one ongoing RCT. Each trial was conducted in a different country: China, Brazil, Egypt, and Japan. We assessed the trials to have an unclear risk of bias for most domains due to insufficient information. Two trials compared intravitreal bevacizumab combined with Ahmed valve implantation and panretinal photocoagulation (PRP) with Ahmed valve implantation and PRP. We did not combine these two trials due to substantial clinical and statistical heterogeneity. One trial randomised participants to receive an injection of either an intravitreal anti-VEGF medication or placebo at the first visit, followed by non-randomised treatment according to clinical findings after one week. The last trial randomised participants to PRP with and without ranibizumab, but details of the study were unavailable for further analysis. Two trials that examined IOP showed inconsistent results. One found inconclusive results for mean IOP between participants who received anti-VEGF medications and those who did not, at one month (mean difference [MD] -1.60 mmHg, 95% confidence interval [CI] -4.98 to 1.78; 40 participants), and at one year (MD 1.40 mmHg, 95% CI -4.04 to 6.84; 30 participants). Sixty-five percent of the participants with anti-VEGF medications achieved IOP ≤ 21 mmHg, versus 60% without anti-VEGF medications. In another trial, those who received anti-VEGF medications were more likely to reduce their IOP than those who did not receive them, at one month (MD -6.50 mmHg, 95% CI -7.93 to -5.07; 40 participants), and at one year (MD -12.00 mmHg, 95% CI -16.79 to -7.21; 40 participants). Ninety-five percent of the participants with anti-VEGF medications achieved IOP ≤ 21 mmHg, versus 50% without anti-VEGF medications. The certainty of a body of evidence was low for this outcome due to limitations in the design and inconsistency of results between studies. Post-operative complications included anterior chamber bleeding (3 eyes) and conjunctival hemorrhage (2 participants) in the anti-VEGF medications group, and retinal detachment and phthisis bulbi (1 participant each) in the control group. The certainty of evidence is low due to imprecision of results and indirectness of evidence. No trial reported the proportion of participants with improvement in visual acuity, proportion of participants with complete regression of new iris vessels, or the proportion of participants with relief of pain and resolution of redness at four- to six-week, or one-year follow-up.
Authors' conclusions: Currently available evidence is uncertain regarding the long-term effectiveness of anti-VEGF medications, such as intravitreal ranibizumab or bevacizumab or aflibercept, as an adjunct to conventional treatment in lowering IOP in NVG. More research is needed to investigate the long-term effect of these medications compared with, or in addition to, conventional surgical or medical treatment in lowering IOP in NVG.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Arathi Simha: none known Kanza Aziz: none known Andrew Braganza: none known Lekha Abraham: none known Prasanna Samuel: none known Kristina Lindsley: none known
Figures
Update of
-
Anti-vascular endothelial growth factor for neovascular glaucoma.Cochrane Database Syst Rev. 2013 Oct 2;10(10):CD007920. doi: 10.1002/14651858.CD007920.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2020 Feb 6;2:CD007920. doi: 10.1002/14651858.CD007920.pub3. PMID: 24089293 Free PMC article. Updated.
References
References to studies included in this review
Arcieri 2015 {published data only}
-
- ACTRN12607000577415. Efficacy and safety of intravitreal avastin in eyes with neovascular glaucoma undergoing Ahmed glaucoma valve implantation. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82392 (first received 4 November 2007).
-
- Arcieri ES, Paula JS, Jorge R, Barella KA, Arcieri RS, Secches DJ, et al. Efficacy and safety of intravitreal bevacizumab in eyes with neovascular glaucoma undergoing Ahmed glaucoma valve implantation: 2‐year follow‐up. Acta Ophthalmologica 2015;93(1):e1‐6. - PubMed
-
- Arcieri ES, Secches DJL, Paula JS, Barella KA, Arcieri RS, Jorge R, et al. Efficacy and safety of intravitreal bevacizumab in eyes with neovascular glaucoma undergoing Ahmed glaucoma valve implantation ‐ preliminary report. ARVO. 2010:627/A471. - PubMed
Jiang 2015 {published data only}
-
- Jiang WP, Lu SS, Jin Y. Clinical research of retinal laser photocoagulation and ranibizumab on the treatment of neovascular glaucoma. International Eye Science 2015;15(10):1763‐5.
Mahdy 2013 {published data only}
-
- Mahdy RA, Nada WM, Fawzy KM, Alnashar HY, Almosalamy SM. Efficacy of intravitreal bevacizumab with panretinal photocoagulation followed by Ahmed valve implantation in neovascular glaucoma. Journal of Glaucoma 2013;22(9):768‐72. - PubMed
NCT02396316 {published data only}
-
- NCT02396316. Japanese phase 3 study of aflibercept in neovascular glaucoma patients. clinicaltrials.gov/ct2/show/NCT02396316 (first received 24 March 2015).
References to studies excluded from this review
Bodla 2017 {published data only}
-
- Bodla AA, Bodla MA. A prospective study analyzing short term effects of intravitreal versus intracameral bevacizumab on neovascular glaucoma. Medical Forum Monthly 2017;28(4):9‐12.
Caujolle 2012 {published data only}
-
- Caujolle JP, Maschi C, Freton A, Pages G, Gastaud P. Treatment of neovascular glaucoma after proton therapy for uveal melanomas with ranibizumab injection: preliminary results. Ophthalmic Research 2012;47(2):57‐60. - PubMed
Chakrabarti 2008 {published data only}
-
- Chakrabarti M, Chakrabarti A, Stephen V, John SR. Efficacy of combining intravitreal bevacizumab monotherapy with panretinal photocoagulation in early stages of neovascular glaucoma. American Academy of Ophthalmology. 2008:182.
ChiCTR‐IPR‐15006695 {published data only}
-
- ChiCTR‐IPR‐15006695. Adjunctive with intravitreal injection of ranibizumab before Ahmed glaucoma valve implantation in the treatment of neovascular glaucoma: a prospective randomized controlled study. www.chictr.org.cn/hvshowproject.aspx?id=11160 (first received 5 July 2015).
Costagliola 2008 {published data only}
Eid 2009 {published and unpublished data}
-
- Eid T, Radwan A, el‐Manawy W, el‐Hawary I. Intravitreal bevacizumab and aqueous shunting surgery for neovascular glaucoma: safety and efficacy. Canadian Journal of Ophthalmology 2009;44(4):451‐6. - PubMed
-
- Eid TM, Radwan A, el‐Menawy W, el‐Hawary I. Outcome of intravitreal bevacizumab (Avastin) followed by aqueous shunting surgery for management of intractable neovascular glaucoma. American Academy of Ophthalmology. 2008:219.
EUCTR2007‐000585‐21‐IE {published data only}
-
- EUCTR2007‐000585‐21‐IE. Randomised controlled trial of intravitreal bevacizumab vs. conventional treatment for rubeotic glaucoma. www.clinicaltrialsregister.eu/ctr‐search/trial/2007‐000585‐21/IE (first received 6 February 2007).
Gupta 2009 {published data only}
-
- Georgalas I, Koutsandrea C, Papaconstantinou D, Petrou P, Ladas I. The effect of different doses of intracameral bevacizumab on surgical outcomes of trabeculectomy for neovascular glaucoma. European Journal of Ophthalmology 2010;20(1):251. - PubMed
-
- Gupta V, Jha R, Rao A, Kong G, Sihota R. The effect of different doses of intracameral bevacizumab on surgical outcomes of trabeculectomy for neovascular glaucoma. European Journal of Ophthalmology 2009;19(3):435‐41. - PubMed
Jonas 2010 {published data only}
-
- Jonas JB, Golubkina L, Libondi T, Rensch F. Intravitreal bevacizumab for neovascular glaucoma. Acta Opthalmologica 2010;88(2):e22‐3. - PubMed
Kong 2017 {published data only}
-
- Kong F, Ma X, Fan S, Lu J, Qin X, Zou J. Efficacy of intravitreal lucentis or conbercept injection combined with Ahmed glaucoma valve implantation for treatment of neovascular glaucoma. Journal of Jilin University (Medicine Edition) 2017;43(6):1237‐42.
Lin 2018 {published data only}
-
- Lin N, Zheng WD, Li B. Clinical effect of ranibizumab combined with PPV on PDR with neovascular glaucoma at early stage. International Eye Science 2018;18(2):294‐7.
Miki 2011 {published data only}
NCT01128699 {unpublished data only}
-
- NCT01128699. Ahmed valve glaucoma implant with adjunctive subconjunctival bevacizumab in refractory glaucoma. clinicaltrials.gov/ct2/show/NCT01128699 (first received 24 May 2010).
NCT01711879 {unpublished data only}
-
- NCT01711879. Use of intravitreal aflibercept injection for neovascular glaucoma. clinicaltrials.gov/ct2/show/NCT01711879 (first received 22 October 2012).
NCT03154892 {published data only}
-
- NCT03154892. The effect of conbercept injection through different routes for neovascular glaucoma. clinicaltrials.gov/ct2/show/NCT03154892 (first received 16 May 2017).
Sedghipour 2011 {published data only}
Silva 2006 {published data only}
-
- Silva Paula J, Jorge R, Alves Costa R, Rodrigues Mde L, Scott IU. Short‐term results of intravitreal bevacizumab (Avastin) on anterior segment neovascularization in neovascular glaucoma. Acta Ophthalmologica Scandinavica 2006;84(4):556‐7. - PubMed
Wang 2016 {published data only}
-
- Wang F, Wang LL. Safety of anti‐VEGF drugs with trabeculectomy for neovascular glaucoma. International Eye Science 2016;16(5):837‐40.
Wittstrom 2012 {published data only}
-
- Wittstrom E, Holmberg H, Hvarfner C, Andreasson S. Clinical and electrophysiologic outcome in patients with neovascular glaucoma treated with and without bevacizumab. European Journal of Ophthalmology 2012;22(4):563‐74. - PubMed
Yazdani 2009 {published data only}
-
- Yazdani S, Hendi K, Pakravan M, Mahdavi M, Yaseri M. Intravitreal bevacizumab for neovascular glaucoma: a randomized controlled trial. Journal of Glaucoma 2009;18(8):632‐7. - PubMed
References to ongoing studies
NCT02914626 {unpublished data only}
-
- NCT02914626. Intravitreal ranibizumab (Lucentis®) for neovascular glaucoma ‐ a randomized controlled study. clinicaltrials.gov/ct2/show/NCT02914626 (first received 26 September 2016).
Additional references
Aiello 1994
-
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. New England Journal of Medicine 1994;331(22):1480‐7. - PubMed
Andreoli 2007
-
- Andreoli CM, Miller JW. Anti‐vascular endothelial growth factor therapy for ocular neovascular disease. Current Opinion in Ophthalmology 2007;18(6):502‐8. [PUBMED: 18163003] - PubMed
Avery 2006
-
- Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina 2006;26(3):352‐4. - PubMed
Blankenship 1988
-
- Blankenship GW. A clinical comparison of central and peripheral argon laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology 1988;95(2):170‐7. - PubMed
Covidence [Computer program]
-
- Veritas Health Innovation. Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 1 May 2019.
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Delgado 2003
-
- Delgado MF, Dickens CJ, Iwach AG, Novack GD, Nychka DS, Wong PC, et al. Long‐term results of noncontact neodymium:yttrium‐aluminum‐garnet cyclophotocoagulation in neovascular glaucoma. Ophthalmology 2003;110(5):895‐9. - PubMed
Doft 1984
-
- Doft BH, Blankenship G. Retinopathy risk factor regression after laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology 1984;91(12):1453‐7. - PubMed
Dong 2018
Ehlers 2008
-
- Ehlers JP, Spirn MJ, Lam A, Sivalingam A, Samuel MA, Tasman W. Combination intravitreal bevacizumab/panretinal photocoagulation versus panretinal photocoagulation alone in the treatment of neovascular glaucoma. Retina 2008;28(5):696‐702. - PubMed
Gandhi 2008
-
- Gandhi JS. Use of antivascular agents for neovascular glaucoma: benefits beyond pressure?. Clinical and Experimental Ophthalmology 2008;36(1):102‐3. - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence prime), accessed 2 January 2019.
Grover 2009
-
- Grover S, Gupta S, Sharma R, Brar VS, Chalam KV. Intracameral bevacizumab effectively reduces aqueous vascular endothelial growth factor concentrations in neovascular glaucoma. British Journal of Ophthalmology 2009; Vol. 93, issue 2:273‐4. - PubMed
Ha 2017
Hayreh 2003
-
- Hayreh SS. Management of central retinal vein occlusion. Ophthalmologica 2003;217(3):167‐88. - PubMed
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hwang 2015
-
- Hwang HB, Han JW, Yim HB, Lee NY. Beneficial effects of adjuvant intravitreal bevacizumab injection on outcomes of Ahmed glaucoma valve implantation in patients with neovascular glaucoma: systematic literature review. Journal of Ocular Pharmacology and Therapeutics 2015;31(4):198‐203. [PUBMED: 25714761] - PubMed
Iliev 2006
-
- Iliev ME, Domig D, Wolf‐Schnurrbursch U, Wolf S, Sarra GM. Intravitreal bevacizumab (Avastin) in the treatment of neovascular glaucoma. American Journal of Ophthalmology 2006;142(6):1054‐6. - PubMed
Kang 2014
-
- Kang JY, Nam KY, Lee SJ, Lee SU. The effect of intravitreal bevacizumab injection before Ahmed valve implantation in patients with neovascular glaucoma. International Ophthalmology 2014;34(4):793‐9. - PubMed
Kiuchi 2006
-
- Kiuchi Y, Nakae K, Saito Y, Ito S, Ito N. Pars plana vitrectomy and panretinal photocoagulation combined with trabeculectomy for successful treatment of neovascular glaucoma. Graefe's Archive for Clinical and Experimental Ophthalmology 2006;244(12):1627‐32. - PubMed
Kovacic 2004
-
- Kovacic Z, Ivanisevic M, Rogosic V, Plavec A, Karelovic D. Cyclocryocoagulation in treatment of neovascular glaucoma [Ciklokriokoagulacija u lijecenju neovaskularnoga glaukoma]. Lijecnicki Vjesnik 2004;126(9‐10):240‐2. - PubMed
Minckler 2006
Noor 2017
Olmos 2016
Pagenstecher 1871
-
- Pagenstecher H. Contribution to the teachings of hemorrhagic glaucoma [Beitrage zur Lehre vom hämorrhagischen Glaucom]. Graefe's Archive for Clinical and Experimental Ophthalmology 1871;17:98‐130.
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Solomon 2019
Sone 1996
-
- Sone H, Okuda Y, Kawakami Y, Hanatani M, Suzuki H, Kozawa T, et al. Vascular endothelial growth factor level in aqueous humor of diabetic patients with rubeotic glaucoma is markedly elevated. Diabetes Care 1996;19(11):1306‐7. - PubMed
Tripathi 1998
-
- Tripathi RC, Li J, Tripathi BJ, Chalam KV, Adamis AP. Increased level of vascular endothelial growth factor in aqueous humor of patients with neovascular glaucoma. Ophthalmology 1998;105(2):232‐7. - PubMed
Tsai 2008
-
- Tsai JC, Wand M. Chapter 213: Neovascular Glaucoma. In: Alm A, Grosskreutz C editor(s). Albert and Jakobiec's Principles and Practice of Ophthalmology. 3rd Edition. Vol. 2, Canada: Saunders Elsevier, 2008:2689.
Wakabayashi 2008
-
- Wakabayashi T, Oshima Y, Sakaguchi H, Ikuno Y, Miki A, Gomi F, et al. Intravitreal bevacizumab to treat iris neovascularization and neovascular glaucoma secondary to ischemic retinal diseases in 41 consecutive cases. Ophthalmology 2008;115(9):1571‐80. - PubMed
Wilkins 2005
Wormald 2001
Yalvac 2007
-
- Yalvac IS, Eksioglu U, Satana B, Duman S. Long‐term results of Ahmed glaucoma valve and Molteno implant in neovascular glaucoma. Eye 2007;21(1):65‐70. - PubMed
Yazdani 2007
-
- Yazdani S, Hendi K, Pakravan M. Intravitreal bevacizumab (Avastin) injection for neovascular glaucoma. Journal of Glaucoma 2007;16(5):437‐9. - PubMed
Zhou 2016
-
- Zhou M, Xu X, Zhang X, Sun X. Clinical outcomes of Ahmed glaucoma valve implantation with or without intravitreal bevacizumab pretreatment for neovascular glaucoma: a systematic review and meta‐analysis. Journal of Glaucoma 2016;25(7):551‐7. [PUBMED: 25719237] - PubMed
References to other published versions of this review
Simha 2009
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials