Two Decades of Successful SAR-Grounded Stories of the Novel Bacterial Topoisomerase Inhibitors (NBTIs)
- PMID: 32027491
- PMCID: PMC7307926
- DOI: 10.1021/acs.jmedchem.9b01738
Two Decades of Successful SAR-Grounded Stories of the Novel Bacterial Topoisomerase Inhibitors (NBTIs)
Abstract
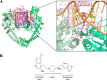
The emergence of bacterial resistance against life-saving medicines has forced the scientific community and pharmaceutical industry to take actions in the quest for novel antibacterials. These should not only overcome the existing bacterial resistance but also provide at least interim effective protection against emerging bacterial infections. Research into DNA gyrase and topoisomerase IV inhibitors has become a particular focus, with the description of a new class of bacterial topoisomerase type II inhibitors known as "novel bacterial topoisomerase inhibitors", NBTIs. Elucidation of the key structural modifications incorporated into these inhibitors and the impact these can have on their general physicochemical properties are detailed in this review. This defines novel bacterial topoisomerase inhibitors with promising antibacterial activities and potencies, which thus represent one potential example of the future "drugs for bad bugs", as identified by the World Health Organization.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
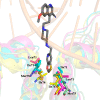

References
-
- World Health Organization. Antimicrobial Resistance: Global Report On Surveillance 2014. http://www.who.int/drugresistance/documents/surveillancereport/en (accessed Aug 7, 2019).
-
- Coates W. J.; Gwynn M. N.; Hatton I. K.; Masters P. J.; Pearson N. D.; Rahman S. S.; Slocombe B.; Warrack J. D.. Preparation Of Piperidinylalkylquinolines As Antibacterials. Patent WO 1999037635, 1999.
-
- Malleron J.-L.; Tabart M.; Carry J. C.; Evers M.; El Ahmad Y.; Mignani S.; Viviani F.. Quinolyl Propyl Piperidine Derivatives And Theiruse As Antibacterial Agents. Patent WO 200125227, 2001.
-
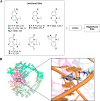
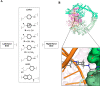
- Bax B. D.; Chan P. F.; Eggleston D. S.; Fosberry A.; Gentry D. R.; Gorrec F.; Giordano I.; Hann M. M.; Hennessy A.; Hibbs M.; Huang J.; Jones E.; Jones J.; Brown K. K.; Lewis C. J.; May E. W.; Saunders M. R.; Singh O.; Spitzfaden C. E.; Shen C.; Shillings A.; Theobald A. J.; Wohlkonig A.; Pearson N. D.; Gwynn M. N. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature 2010, 466, 935–940. 10.1038/nature09197. - DOI - PubMed
-
- Surivet J. P.; Zumbrunn C.; Rueedi G.; Hubschwerlen C.; Bur D.; Bruyère T.; Locher H.; Ritz D.; Keck W.; Seiler P.; Kohl C.; Gauvin J. C.; Mirre A.; Kaegi V.; Dos Santos M.; Gaertner M.; Delers J.; Enderlin-Paput M.; Boehme M. Design, synthesis, and characterization of novel tetrahydropyran-based bacterial topoisomerase inhibitors with potent anti-Gram-positive activity. J. Med. Chem. 2013, 56, 7396–7415. 10.1021/jm400963y. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Research Materials
Miscellaneous

