Exosomes mediate sensory hair cell protection in the inner ear
- PMID: 32027617
- PMCID: PMC7190999
- DOI: 10.1172/JCI128867
Exosomes mediate sensory hair cell protection in the inner ear
Abstract
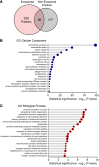
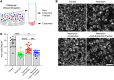
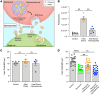
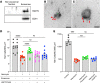
Hair cells, the mechanosensory receptors of the inner ear, are responsible for hearing and balance. Hair cell death and consequent hearing loss are common results of treatment with ototoxic drugs, including the widely used aminoglycoside antibiotics. Induction of heat shock proteins (HSPs) confers protection against aminoglycoside-induced hair cell death via paracrine signaling that requires extracellular heat shock 70-kDa protein (HSP70). We investigated the mechanisms underlying this non-cell-autonomous protective signaling in the inner ear. In response to heat stress, inner ear tissue releases exosomes that carry HSP70 in addition to canonical exosome markers and other proteins. Isolated exosomes from heat-shocked utricles were sufficient to improve survival of hair cells exposed to the aminoglycoside antibiotic neomycin, whereas inhibition or depletion of exosomes from the extracellular environment abolished the protective effect of heat shock. Hair cell-specific expression of the known HSP70 receptor TLR4 was required for the protective effect of exosomes, and exosomal HSP70 interacted with TLR4 on hair cells. Our results indicate that exosomes are a previously undescribed mechanism of intercellular communication in the inner ear that can mediate nonautonomous hair cell survival. Exosomes may hold potential as nanocarriers for delivery of therapeutics against hearing loss.
Keywords: Apoptosis survival pathways; Cell Biology; Neurodegeneration; Neuroscience.
Conflict of interest statement
Figures




Comment in
-
Exosome-mediated protection of auditory hair cells from ototoxic insults.J Clin Invest. 2020 May 1;130(5):2206-2208. doi: 10.1172/JCI135710. J Clin Invest. 2020. PMID: 32310224 Free PMC article.
References
-
- World Health Organization. WHO Global Estimates on Prevalence of Hearing Loss. http://www.who.int/deafness/estimates/en Accessed March 6, 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

