Impact of the BioFire FilmArray gastrointestinal panel on patient care and infection control
- PMID: 32027698
- PMCID: PMC7004333
- DOI: 10.1371/journal.pone.0228596
Impact of the BioFire FilmArray gastrointestinal panel on patient care and infection control
Abstract
Objectives: Conventional routine PCR testing for gastrointestinal infections is generally based on pathogen related panels specifically requested by clinicians and can be erroneous and time consuming. The BioFire FilmArray gastrointestinal (GI) panel combines 22 pathogens into a single cartridge-based test on a random-access system, thereby reducing the turnaround time to less than 2 hours. We described the clinical impact of implementing the BioFire FilmArray on patients with gastroenteritis in our hospital.
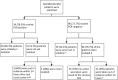
Methods: Patients attending a Dutch tertiary care center (Radboud University Medical Center), from whom stool samples were obtained, were eligible for inclusion. The clinicians selected one or a combination of different routinely performed PCR panels (bacterial panel, viral panel, clostridium testing, and three parasitic panels) based on clinical history and symptoms. All samples were in parallel tested with the FilmArray. We retrospectively collected patient data regarding infection control and patient management to assess the potential impact of implementing the FilmArray.
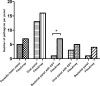
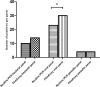
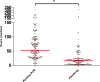
Results: In total 182 patients were included. Routine PCR detected one or more pathogens in 52 (28.6%) patients compared to 72 (39.6%) using the FilmArray. Turnaround time (including transport) decreased from median 53 hours for the routine PCR to 16 hours for the FilmArray. Twenty-six patients could have been removed from isolation 29 hours sooner, 3.6 antibiotic days could have been saved and in five patients additional imaging testing (including colonoscopies) could have been prevented.
Conclusion: The theoretical implementation of the BioFire FilmArray GI panel in patients with clinical suspicion of gastroenteritis resulted in a significant better patient management.
Conflict of interest statement
BioFire Diagnostics provided the system and the cartridges to perform the tests. The authors declare that there are no conflicts of interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Bos J., Schultsz C., Van Gool T., Bauer M., and Prins J., Swab guideline antimicrobial therapy in acute infectious diarrhea. 2014.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

